Diabetes mellitus type 2
Diabetes mellitus type 2 is a "subclass of diabetes mellitus that is characterized initially by insulin resistance and hyperinsulinemia; and eventually by glucose intolerance; hyperglycemia; and overt diabetes. Type II diabetes mellitus is no longer considered a disease exclusively found in adults. Patients seldom develop ketosis but often exhibit obesity."[1]
The U.S. Centers for Disease Control and Prevention provides a more expanded description:[2]
Type 2 diabetes was previously called non–insulin-dependent diabetes mellitus (NIDDM) or adult-onset diabetes. In adults, type 2 diabetes accounts for about 90% to 95% of all diagnosed cases of diabetes. It usually begins as insulin resistance, a disorder in which the cells do not use insulin properly. As the need for insulin rises, the pancreas gradually loses its ability to produce it. Type 2 diabetes is associated with older age, obesity, family history of diabetes, history of gestational diabetes, impaired glucose metabolism, physical inactivity, and race/ethnicity. African Americans, Hispanic/Latino Americans, American Indians, and some Asian Americans and Native Hawaiians or Other Pacific Islanders are at particularly high risk for type 2 diabetes and its complications. Type 2 diabetes in children and adolescents, although still rare, is being diagnosed more frequently among American Indians, African Americans, Hispanic/Latino Americans, and Asians/Pacific Islanders. [3]
Impaired glucose metabolism refers to impaired fasting glucose and impaired glucose tolerance.
Clinical practice guidelines address the care of patients with diabetes mellitus type 2.[4]
Prevalence
United States
|
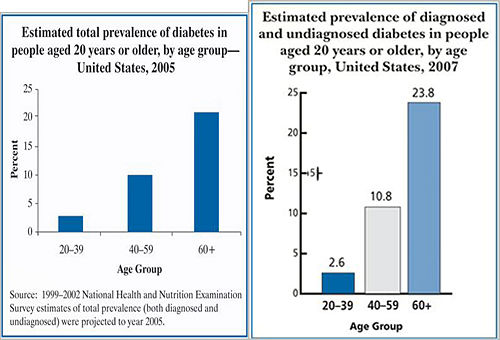
Prevalence of Diagnosed and Undiagnosed Diabetes Among People Aged 20 Years or Older, United States, 2007 [5] |
|
|
Etiology
Diabetes mellitus type 2 is characterized by insulin resistance, high insulin levels, and declining function of insulin-secreting cells of the pancreatic islets (beta-cells).
Diabetes mellitus type 2 is associated with the Metabolic syndrome X (Abdominal obesity-metabolic syndrome).[6]
Genetics
Diabetes mellitus type 2 is genetically heterogeneous and variations are labeled NIDDM2, NIDDM3, NIDDM4.[7]
Maturity-onset diabetes of the young (MODY)
Maturity-onset diabetes of the young (MODY) is "an autosomal dominant form of diabetes typically occurring before 25 years of age and caused by primary insulin secretion defects. Despite its low prevalence, MODY is not a single entity but represents genetic, metabolic, and clinical heterogeneity"[8] Several mutations may cause MODY and these variations are labeled MODY1, MODY2, ... MODY9. Because these mutations may cause diabetes at a later ge and other conditions can cause early diabetes, it has been suggestions to label these mutations "autosomal dominant noninsulin-dependent."[9]
Diagnosis
The World Health Organization definition of diabetes is for a single raised glucose reading with symptoms, otherwise raised values on two occasions, of either[10]:
- Fasting plasma glucose ≥ 7.0mmol/l (126mg/dl)
- or
- With a Glucose tolerance test, two hours after the oral dose a plasma glucose ≥ 11.1mmol/l (200mg/dl)
An alternative algorithm that diagnoses diabetes with high sensitivity and specificity:[11]
- Fasting plasma glucose ≥ 7.0mmol/l (126mg/dl)
- Diabetes is diagnosed
- or
- Glycosylated hemoglobin A (Hb A1c) ≥ 7.0
- Refer for oral glucose tolerance test
Glycosylated hemoglobin A
HbA1c levels 5.5% may rule out while levels 7.0% may rule in diabetes.[12]
Regarding a single cutoff, a glycosylated hemoglobin A value of ≥ 6.5% may be used to diagnose.[13][14]
Impaired fasting glucose
Impaired fasting glucose is defined as:[10][15]
- Fasting glucose level > 5.6 mmol/l (100 mg/dl) and < 6.9 mmol/l (125mg/dl).
Impaired glucose tolerance
Impaired glucose tolerance is defined as[10][15]:
- Two-hour glucose levels of 140 to 199 mg per dL (7.8 to 11.0 mmol) on the 75-g oral glucose tolerance test
Screening and prevention
A cost-benefit analysis suggests that screening should start between age 30 and 45 and be repeated every 3 to 5 years.[16]
Clinical practice guidelines
US Preventive Services Task Force
Regarding the mass screening for diabetes, in 2014 the U.S. Preventive Services Task Force draft recommendations were:[17]
- Appropriate tests are "measuring hemoglobin A1c or fasting plasma glucose or with an oral glucose tolerance test"
- Regarding who to screen: "Risk factors include age of 45 years or older, overweight or obesity, or a first-degree relative with diabetes. Women with a history of gestational diabetes or polycystic ovarian syndrome are also at increased risk. Certain racial/ethnic minorities, including African Americans, American Indians/Alaska Natives, Asian Americans, Hispanics/Latinos, and Native Hawaiians/Pacific Islanders, are also at increased risk compared with whites."
- Regarding confirmation of positive screening: "The diagnosis of IFG, IGT, and type 2 diabetes must be confirmed unless symptoms are present. Repeat testing using the same test on a different day is the preferred method of confirmation. If diagnosis cannot be confirmed by the results of two tests, but at least one test indicates high risk, clinicians may wish to follow the patient closely and retest in 3 to 6 months"
- Regarding rescreening: "every 3 years may be a reasonable approach for adults at low risk with normal blood glucose levels. For adults at high risk or those who have near abnormal test values, repeat annual screening may be warranted."
Prior recommendations
In 2008 the U.S. Preventive Services Task Force concluded:[18] [19]
- "Screen for type 2 diabetes in asymptomatic adults with sustained blood pressure (either treated or untreated) greater than 135/80 mm Hg. (B recommendation)"
- "Current evidence is insufficient to assess the balance of benefits and harms of routine screening in asymptomatic adults with blood pressure of 135/80 mm Hg or lower. (I statement)"
Screening obese patients may also be beneficial.[20]
In 2003, U.S. Preventive Services Task Force concluded that the benefits are:[21][22]
- In hypertensive patients, identifying diabetes would lower the goal diastolic pressure to ≤ 80 mm Hg.
- In hypercholesterolemia patients, identifying diabetes would affect decision making due to changes in calculating cardiovascular risk in the ATP3 clinical practice guideline.[23]
The USPSTF recommended:
- "The USPSTF recommends screening for type 2 diabetes in adults with hypertension or hyperlipidemia." This was a grade B recommendation
- "The evidence is insufficient to recommend for or against routinely screening asymptomatic adults for type 2 diabetes, impaired glucose tolerance, or impaired fasting glucose" (italics by CZ), this was a grade I recommendation when published in 2003.
Canadian Task Force on Preventive Health Care
The Canadian Task Force on Preventive Health Care recommends:[24] Based on Finnish Diabetes Risk Score else Canadian Diabetes Risk Assessment Questionnaire], frequency of screening with glycosylated hemoglobin A:
- Very high risk or prediabetes (50% within 10 years) - Finnish Diabetes Risk Score > 20: screen annually
- High risk (33% within 10 years) - Finnish Diabetes Risk Score 15 to 20: screen every 3–5 years
Other practice guidelines
In 2005, an evidence report by the Agency for Healthcare Research and Quality (AHRQ) concluded that "there is evidence that combined diet and exercise, as well as drug therapy (metformin, acarbose), may be effective at preventing progression to DM in IGT subjects".[25]
The American College of Endocrinology announced in 2008 clinical practice guidelines for the treatment of prediabetes.[26]
Accuracy of tests for early detection
Various testing strategies are available[27][28], including [[clinical prediction rule]s].[29][30]
- Fasting plasma glucose
The fasting plasma glucose > 7.0 mmol/L (126 mg/dL), compared to a 2-hour postload glucose level of at least 11.1 mmol/L (≥ 200 mg/dL) as a reference standard, has[22]:
- sensitivity about 50%
- specificity greater than 95%
- Random capillary blood glucose
A random capillary blood glucose > 6.7 mmol/L (120 mg/dL) has:
- sensitivity of 40%[31] to 75%[32]
- specificity of 93%[31] to 88%[32]
- Glycosylated hemoglobin
Glycosylated hemoglobin A (Hb A1c) values that are elevated (over 5%), but not in the diabetic range (not over 7.0%) are predictive of subsequent clinical diabetes in US female health professionals.[33][34] In this study, 177 of 1061 patients with Hb A1c value less than 6% became diabetic within 5 years compared to 282 of 26281 patients with a Hb A1c value of 6.0% or more. This equates to a Hb A1c value of 6.0% or more having:
- sensitivity = 16.7%
- specificity = 98.9%
Glycosylated hemoglobin A values are not perfect.[35]
- Clinical prediction rule
A clinical prediction rule based on age, gender, family medical history, hypertension, body mass index, and physical activity predicted diabetes with accuracy of:[29]
- sensitivity = 79%
- specificity = 67%
A second clinical prediction rule, QDSCORE®, may "predict the 10-year risk of developing Type 2 diabetes in the UK".[30]
Benefit of early detection
| Study/year | Population | Intervention | Comparison | Outcomes | Results |
|---|---|---|---|---|---|
| ADDITION-Europe Study 2011[36] Cluster trial |
3057 patients • Diabetes diagnosed by mass screening |
Multifactorial interventions aimed at: • Health care providers • Patients |
Usual care | • Glycosylated hemoglobin A • Clinical endpoints |
Hazard ratio 0.83, statistically insignificant |
| NAVIGATOR Study 2010[37] |
9306 patients • Impaired glucose tolerance • Coronary heart disease or risk factors |
Valsartan up to 160 mg daily for five years | Placebo | Diagnosis of diabetes mellitus type 2 | "relative reduction of 14% in the incidence of diabetes but did not reduce the rate of cardiovascular events" |
| NAVIGATOR Study 2010[38] |
9306 patients • Impaired glucose tolerance • Coronary heart disease or risk factors |
Nateglinide up to 60 mg three times daily for five years | Placebo | Diagnosis of diabetes mellitus type 2 | "did not reduce the incidence of diabetes or the coprimary composite cardiovascular outcome" |
| Finnish Diabetes Prevention Study 2001[39] 2006[40] |
522 patients • 40 to 65 years old • BMI at least 25 • Impaired glucose tolerance |
Lifestyle changes for 3.2 years: • diet • exercise |
General information about diet and exercise at given at base line and annually | Diagnosis of diabetes mellitus type 2 | "Type 2 diabetes can be prevented by changes in the lifestyles of high-risk subjects"[39] • "Lifestyle intervention in people at high risk for type 2 diabetes resulted in sustained lifestyle changes and a reduction in diabetes incidence, which remained after the individual lifestyle counseling was stopped."[40] |
| Diabetes Prevention Program (DPP) 2002[47][41][42] |
3234 patients • 30 or more years old • Impaired glucose tolerance or impaired fasting glucose |
Metformin (850 mg twice daily) for 2.8 years | Placebo | • Death or diagnosis of diabetes mellitus type 2[41] • Vascular disease events[42] |
• "Lifestyle changes and treatment with metformin both reduced the incidence of diabetes in persons at high risk" • Did not reduce vascular disease |
| STOP-NIDDM 2003[43] |
1429 patients • 40 to 70 more years old • Impaired glucose tolerance • BMI 25 - 40 |
Acarbose 100 mg of acarbose 3 times a day | Placebo | Vascular disease events | "This study suggests that treating IGT patients with acarbose is associated with a significant reduction in the risk of cardiovascular disease and hypertension" |
| DREAM 2006[44] |
5269 patients • 30 or more years old • Impaired glucose tolerance or impaired fasting glucose |
Rosiglitazone (8 mg per day) for 3 years | Placebo | • Death or diagnosis of diabetes mellitus type 2 • Vascular disease events • Carotid ultrasonography (results not reported)[48] |
• "Rosiglitazone at 8 mg daily for 3 years substantially reduces incident type 2 diabetes and increases the likelihood of regression to normoglycaemia" • Rosiglitazone did not significantly decrease death • Rosiglitazone increased heart failure • Rosiglitazone did not reduce vascular disease |
| DREAM 2006[44] |
5269 patients • 30 or more years old • Impaired glucose tolerance or impaired fasting glucose |
Ramipril (up to 15 mg per day) for 3 years | Placebo | Death or diagnosis of diabetes mellitus type 2 • Vascular disease events |
"ramipril for 3 years does not significantly reduce the incidence of diabetes or death but does significantly increase regression to normoglycemia" |
| Salpeter meta-analysis 2008[45] |
4570 patients in 31 randomized controlled trials including Diabetes Prevention Program Research Group[41] | Metformin | Placebo or no treatment | Varioius outcomes | Metformin treatment in persons at risk for diabetes improves weight, lipid profiles, and insulin resistance, and reduces new-onset diabetes by 40%. |
| Voglibose Ph-3 Study 2009[46] |
1780 patients • 30 to 70 years old • Impaired glucose tolerance • At least one risk factor for diabetes |
Voglibose 0.2 mg three times a day orally | Placebo | Diagnosis of diabetes mellitus type 2 | "Voglibose, in addition to lifestyle modification, can reduce the development of type 2 diabetes in high-risk Japanese individuals with impaired glucose tolerance." |
Treatment
Treatment goals
Remission
In selected patients, dieting to loose approximately 3% of body weight can remit mild, assymptomatic diabetes in approximately 50% of patients according to one trial.[49]
Intensive insulin treatment with a goal of normoglycaemia can cause diabetes in newly diagnosed patients to remit in one of four patients after one year of follow-up.[50] However, there was no control group that did not receive intensive therapy.
Prior uncontrolled cases series have reported similar results.[51][52][53][54]
These studies have been summaried by Retnakaran.[55]
Chronic care of outpatients
For most patients the goal of treatment should be a Hb A1c of 7.0%. The benefit of intensive therapy is not certain.[56] Below is a summary of clinical practice guidelines and randomized controlled trials that support this recommendation.
In older patients with life expectancy of less than 5 years:
- The benefit of achieving a Hb A1c of 7.0% is small according to a decision analysis.[57]
- An Hb A1c of 8.0% - 9.0% may actually be safer according to a cohort study.[58]
Practice guidelines
For most patients, clinical practice guidelines recommend a goal Hb A1c of 7.0%[59][60][61].
Previously the American Diabetic Association (ADA) clinical practice guideline suggested a goal of 6.0%:[62]
- "The A1C goal for patients in general is an A1C goal of <7%."
- "The A1C goal for the individual patient is an A1C as close to normal (<6%) as possible without significant hypoglycemia."
The current ADA recommendation is from a consensus statement of 7% following the results of the trials below that suggest no benefit from goals below 7%.[63]
Clinical practice guidelines by the National Institute for Health and Clinical Excellence recommend starting metformin or a sulfonylurea is Hb A1c is more than 6.5%; however, a thiazolidinedione or insulin should not be added unless the Hb A1c is more than 7.5%.[64][65] These guidelines have been summarized.[66]
In older patients, clinical practice guidelines by the American Geriatrics Society states "for frail older adults, persons with life expectancy of less than 5 years, and others in whom the risks of intensive glycemic control appear to outweigh the benefits, a less stringent target such as 8% is appropriate."[67]
Evidence from trials
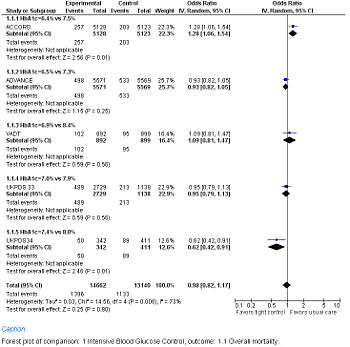
Forest Plot showing meta-analysis of randomized controlled trials of differing target glucose control and mortality for diabetes mellitus type 2. Note the heterogeneity due to increased death when the glycosylated hemoglobin A (Hb A1c) target was 6.0% in the ACCORD trial
A goal fasting blood glucose of below 108 mg/dl (6 mmol/L) over 10 years resulting in an Hb A1c of 7% over 10 years was found in the United Kingdom Prospective Diabetes Study (UKPDS 33) randomized controlled trial. Intensive control reduced diabetic complications in one out of every 20 patients (number needed to treat = 20).[68]
A goal fasting blood glucose of below 108 mg/dl (6 mmol/L) over 10 years resulting in an Hb A1c of 7.4% over 10.7 years in the metformin group compared to 8.0% in the conventional group in the UK Prospective Diabetes Study (UKPDS 34) randomized controlled trial. Metformin reduced cardiovascular disease in one out of every 11 patients (number needed to treat = 11).[69]
A Hb A1c of 6.9% over 6 years was found in the VA Diabetes Trial (VADT) randomized controlled trial to have no significant effect on diabetic complications.[70] Although the treatment group averaged an Hb A1c of 6.9%, the goal was 6.0%.[71]
A Hb A1c goal of 6.5% over 5 years was found in the ADVANCE randomized controlled trial not to reduce mortality using a protocol whose first step was a sulfonylurea (gliclazide). The intervention group had 0.9% less nephropathy, but more severe hypoglycemia.[72]
A Hb A1c goal of 6% over 3.5 years was found in the ACCORD randomized controlled trial found to increase serious complications.[73][74]
The ORIGIN trial used basal insulin supplementation to reduce the Hb A1c from 6.4% to 6.2%. There was no benefit on cardiac outcomes but there was an increase in hypoglycemia and weight gain.[75]
The PROACTIVE study used pioglitazone.[76]
The older University Group Diabetes Program (UGDP) also found no benefit in a controversial randomized controlled trial.[77][78][79][80][81] The UGDP randomized approximately 1000 patients to one of five treatment groups and followed from 1962 to 1975: phenformin, tolbutamide, small fixed-dose insulin (ISTD) based on body-surface area (averaged 14 units per day), variable-dose insulin (ISTD) (averaged 45 units per day), n (IVAR), or placebo. The trial found statistically significant increase in cardiovascular deaths among the patients treated with tolbutamide and so this group was stopped in 1969. The phenformin group was also stopped early due to increased mortality. The ISTD group had no reduction in blood glucose. The IVAR group had a reduction in the IVAR group of about 2.0 mmol/L (36 mg/dL) which correlates to a 1% difference in the level of Hb A1c.[82] Problems in the trial include: 1) "25% of placebo and tolbutamide-treated subjects dropped out or changed medication during the trial[79], 2) glucose values were only checked quarterly[80], 3) smoking history was not measured[80] 4) reduced fraction of males in the IVAR group (IVAR=22%; placebo=31%).
Inpatients
Clinical practice guidelines are available by the American College of Physicians (ACP) and a consensus group[83] are available. The ACP recommends:[84]
- "ACP recommends not using intensive insulin therapy to strictly control blood glucose in non–surgical intensive care unit (SICU)/medical intensive care unit (MICU) patients with or without diabetes mellitus (Grade: strong recommendation, moderate-quality evidence)."
- "ACP recommends not using intensive insulin therapy to normalize blood glucose in SICU/MICU patients with or without diabetes mellitus (Grade: strong recommendation, high-quality evidence)."
- "ACP recommends a target blood glucose level of 7.8 to 11.1 mmol/L (140 to 200 mg/dL) if insulin therapy is used in SICU/MICU patients (Grade: weak recommendation, moderate-quality evidence)."
This recommendation was updated without changes in 2013.[85]
Intensive care
Two clinical practice guidelines are available; however, both of these guidelines were developed without broad representation of stakeholders.[86] This may lead to overly aggressive clinical recommendations.
A clinical practice guideline from the American Association of Clinical Endocrinologists (AACE) recommends the following target blood glucose levels:[87]
- "Critically ill patients, between 80 to 110 mg/dL (grade A recommendation)"
A clinical practice guideline from the American Diabetes Association (ADA) states[61]
- "Critically ill patients: blood glucose levels should be kept as close to 110 mg/dl (6.1 mmol/l) as possible and generally <140 mg/dl (7.8 mmol/l). (A) These patients require an intravenous insulin protocol that has demonstrated efficacy and safety in achieving the desired glucose range without increasing risk for severe hypoglycemia. (E)"
Randomized controlled trials of tight glucose control in the critical care and perioperative care settings have produced mixed results. A meta-analysis of trials in the critical care setting concludes there is no benefit to tight control.[88]
Since the meta-analysis, two negative randomized controlled trials have been published.[89][90]
Non-intensive care
Two clinical practice guidelines are available; however, both of these guidelines were developed without broad representation of stakeholders.[86] This may lead to overly aggressive clinical recommendations. A meta-analysis has addressed this issue.[91]
A clinical practice guideline from the American Association of Clinical Endocrinologists (AACE) recommends the following target blood glucose levels:[87]
- "Preprandial, less than 110 mg/dL (grade C recommendation)"
- "Peak postprandial, less than 180 mg/dL (grade B recommendation)"
A clinical practice guideline from the American Diabetes Association (ADA) states[61]
- "Non–critically ill patients: there is no clear evidence for specific blood glucose goals. Since cohort data suggest that outcomes are better in hospitalized patients with fasting glucose <126 mg/dl and all random glucoses <180–200, these goals are reasonable if they can be safely achieved. Insulin is the preferred drug to treat hyperglycemia in most cases. (E)"
- "Due to concerns regarding the risk of hypoglycemia, some institutions may consider these blood glucose levels to be overly aggressive for initial targets. Through quality improvement, glycemic goals should systematically be reduced to the recommended levels. (E)"
Older versions (2007) of these clinical practice guidelines were more aggressive regarding non-critically ill patients:
- "Non-critically ill patients: premeal blood glucose levels should be kept as close to 90 to 130 mg/dL (5.0 to 7.2 mmol/L; midpoint of range 110 mg/dL) as possible given the clinical situation and postprandial blood glucose levels <180 mg/dL. Insulin should be used as necessary. (E)"
Intraoperative care
Regarding [[perioperative care|intraoperative control of glucose, a randomized controlled trial concluded "the increased incidence of death and stroke in the intensive treatment group raises concern about routine implementation of this intervention."[92]
Pediatrics
Lower blood sugars may be beneficial in the intensive care of children.[93]
Self monitoring of blood glucose
It is unclear if self-monitoring of blood glucose improves outcomes[94], especially among "reasonably well controlled non-insulin treated patients with type 2 diabetes."[95] Self-monitoring may reduce quality of life.[96]
Diet
A low glycemic index diet may reduce the HbA1c.[97]
Available classes of antidiabetic drugs
Sulfonylureas
Sulfonylureas are insulin secretagogues. They cause weight gain.
- Glipizide is mainly excreted by the liver
- Glyburide is excreted by both liver and kidneys.
Meglitinides
Meglitinides stimulate insulin release. Examples include nateglinide, repaglinide, and their analogs. These are associated with weight gain.[98]
Biguanides
Biguanides are insulin sensitizers and include metformin and phenformin.
SGLT2 inhibitors
SGLT2 inhibitors (Gliflozin) inhibit renal sodium-glucose transport protein 2 (SGLT2)leading reduced reabsorption of glucose in the kidney. Examples include dapagliflozin, empagliflozin, canagliflozin.
Thiazolidinediones
Thiazolidinediones (TZDs) are insulin sensitizers and include rosiglitazone, pioglitazone, and troglitazone. Rosiglitazone is safer than pioglitazone according to a systematic review published in 2011.[99]
α-glucosidase inhibitors
α-glucosidase inhibitors reduce carbohydrate absorption and include acarbose and miglitol.
Peptide analogs
In general, these do not cause weight gain.[98]
Incretin therapy can reduce the glycosylated hemoglobin[100] but may increase cancer[101][102] and pancreatitis[101].
- Incretin mimetics. Incretin is an insulin secretagogue.
- Glucagon-like peptide (GLP) analogs (subcutaneous administration)
- Exenatide (Byetta®,Bydureon®) which can be given twice daily (Byetta®) or once weekly (Bydureon®).[103]
- Liraglutide (Victoza®) may be more effective than the peptide analog sitagliptin because GLP-1 receptor agonists do not rely on endogenous incretin secretion that DPP-4 inhibitors require.[104]
- Gastric inhibitory peptide (GIP) analogs
- None are FDA approved
- Glucagon-like peptide (GLP) analogs (subcutaneous administration)
- Incretin enhancement by dipeptidyl peptidase-4 (DPP-4) inhibitors[105]
- Alogliptin (SYR-322) is an oral inhibitor of DPP-4[106]
- Saxagliptin (Onglyza®) is taken orally
- Sitagliptin (Januvia®) is taken orally
Amylin therapy
Insulins
Selecting an antidiabetic drug
Oral drugs
If dietary changes are not successful, medication is needed.
Metformin is usually the first drug according to clinical practice guidelines.[59] Metformin and second-generation sulfonylureas and are excellent choices according to a systematic review of randomized controlled trials.[107] Confirming the role of metformin, the initial choice of anti-diabetic drug has been compared in a randomized controlled trial which found "cumulative incidence of monotherapy failure at 5 years of 15% with rosiglitazone, 21% with metformin, and 34% with glyburide."[108] While thiazolidinediones such as rosiglitazone are effective, they may increase drug toxicity such as heart failure and fractures.
For patients with heart failure, metformin may be the best choice.[109]
Insulin regimens
If antidiabetic drugs fail, insulin therapy may be necessary. The initial insulin regimen can be chosen based on the patient's blood glucose profile.[110] When insulin is started, "insulin secretagogues (sulfonylurea or glinides) should be discontinued, or tapered and then discontinued, since they are not considered to be synergistic."[59]
Bedtime insulin
Initially, adding bedtime insulin to patients failing oral medications is more effective and with less weight gain than using multiple dose insulin.[111] Nightly insulin combines better with metformin that with sulfonylureas.[112] The initial dose of nightly insulin (measured in IU/d) should be equal to the fasting blood glucose level (measured in mmol/L). If the fasting glucose is reported in mg/dl, multiple by 0.05551 (or divided by 18) to convert to mmol/L.[113]
Multiple dose insulin
When nightly insulin is insufficient, multiple doses are required.
Typical total daily dosage of insulin is 0.6 U/kg with starting doses of 0.25 U/kg.[111][112] 4 units can be added for each 18 mg/dl over 180 mg/dl.[111] A typical final dose is about 45 units per day.[111] More complicated estimations to guide initial dosage of insulin are:[114]
- For men, [(fasting plasma glucose [mmol/liter]–5)x2] x (weight [kg]÷(14.3xheight [m])–height [m])
- For women, [(fasting plasma glucose [mmol/liter]–5)x2] x (weight [kg]÷(13.2xheight [m])–height [m])
- Premixed insulin with a fixed ratio of short and intermediate acting insulin; this tends to be more effective than long acting insulin, but is associated with more hypoglycemia.[115][116][117]. Initial total daily dosage of biphasic insulin can be 10 units if the fasting plasma glucose values are less than 180 mg/dl or 12 units when the fasting plasma glucose is above 180 mg/dl".[116] A guide to titrating fixed ratio insulin is available (http://www.annals.org/cgi/content/full/145/2/125/T4).[110]
- Long acting insulins such as insulin glargine and insulin detemir (shorter half-life). A meta-analysis of randomized controlled trials by the Cochrane Collaboration found "only a minor clinical benefit of treatment with long-acting insulin analogues for patients with diabetes mellitus type 2."[118] More recent randomized controlled trials have found no differences with glargine.[119] The 4T study reported at 1 year and have found that although long acting insulins were less effective, they were associated with less hypoglycemia.[115] After three years, improved control occurred with the newer insulins with less hypoglycemia.[120]
Treatment of associated diseases
Treating to a goal of LDL-C < 70 mg/dl and systolic blood pressure to < 115 mm Hg may cause regression of carotid initial media thickness in a randomized controlled trial.[121]
Aspirin
Aspirin may not be justified just because a patients had diabetes.[122]
Angiotensin-converting enzyme inhibitors
The HOPE study suggests that diabetics should be treated with angiotensin-converting enzyme inhibitors (specifically ramipril 10 mg/d) if they have one of the following [123]:
- hypertension
- hypercholesterolemia or reduced low high-density lipoprotein cholesterol levels
- cigarette smoking
- microalbuminuria
After treatment with ramipril for 5 years the number needed to treat was 50 patients to prevent one cardiovascular death.
Initially, other angiotensin-converting enzyme inhibitors were thought to be not be as effective.[124] However, subsequent studies have shown the apparent benefit of ramipril independent of blood pressure reduction was more likely due to the administration of ramipril at night and recording blood pressures during the day when the least effect of ramipril was present.[125][126]
Hypertension
| Trial | Population | Intervention | Comparison | Outcome | Comments |
|---|---|---|---|---|---|
| ACCORD[127] 2010 |
4,733 patients | Systolic pressure goal of less than 120 mm Hg for 4.7 years | Systolic pressure goal of less than 140 mm Hg | Mortality | Insignificantly increased |
| ADVANCE[128] 2007 |
11,140 patients | Fixed combination of perindopril and indapamide that achieved blood pressure of 136/73 for 4.3 years | Achieved blood pressure of 130/73 | Mortality | Significantly reduced |
According to clinical practice guidelines, when treating hypertension in the diabetic patients, the goal blood pressure is 130/80 which is lower than in non-diabetic patients.[129] However, a subsequent randomized controlled trial found no benefit in treating to a level of 120 mm Hg.[127]
"In subjects with type 2 diabetes and hypertension but with normoalbuminuria, the use of trandolapril plus verapamil and trandolapril alone decreased the incidence of microalbuminuria to a similar extent. The effect of verapamil alone was similar to that of placebo," according to the BENEDICT randomized controlled trial.[130]
Hypercholesterolemia
Various clinical practice guidelines have addressed the treatment of hypercholesterolemia. The American College of Physicians has addressed hypercholesterolemia in patients with diabetes [131][132]. Their recommendations are:
- Recommendation 1: Lipid-lowering therapy should be used for secondary prevention of cardiovascular mortality and morbidity for all patients (both men and women) with known coronary artery disease and type 2 diabetes.
- Recommendation 2: Statins should be used for primary prevention against macrovascular complications in patients (both men and women) with type 2 diabetes and other cardiovascular risk factors.
- Recommendation 3: Once lipid-lowering therapy is initiated, patients with type 2 diabetes mellitus should be taking at least moderate doses of a statin (the accompanying evidence report states "simvastatin, 40 mg/d; pravastatin, 40 mg/d; lovastatin, 40 mg/d; atorvastatin, 20 mg/d; or an equivalent dose of another statin")[132].
- Recommendation 4: For those patients with type 2 diabetes who are taking statins, routine monitoring of liver function tests or muscle enzymes is not recommended except in specific circumstances.
Statin therapy prevents major vascular events in about 1 of every 24 patients with [diabetes who use the treatment for 5 years if they are similar to the patients in the meta-analysis by Kearney et al (Number needed to treat is 24).[133]
Whether diabetes is an equivalent risk factor to having an existing myocardial infarction is debated.[134]
Treating to a goal of LDL-C < 70 mg/dl and systolic blood pressure to < 115 mm Hg may cause regression of carotid initial media thickness in a randomized controlled trial.[135]
Obesity
Bariatric surgery remits diabetes mellitus type 2 in more than 1 of every two people after 2 years if they are similar to the patients in the randomized controlled trial / meta-analysis by Dixon et al (Number needed to treat is 1.74).[136] In this trial 73% of the patients who remitted their diabetes versus 13% of the patients in the control group.
Complications
Hypoglycemia
Hypoglycemia from intensive therapy is associated with cardiovascular adverse outcomes.[137]
Diabetic foot
Neuropathy
Nephropathy
Hyperglycemic hyperosmolar nonketotic coma
Diabetic ketoacidosis
References
- ↑ Anonymous (2025), Diabetes Mellitus, Type 2 (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ National Center for Chronic Disease Prevention and Health Promotion. (2008) What is Diabetes?
- ↑ National Center for Chronic Disease Prevention and Health Promotion. (2008) What is Diabetes?
- ↑ American Diabetes Association (2011). "Standards of medical care in diabetes--2011.". Diabetes Care 34 Suppl 1: S11-61. DOI:10.2337/dc11-S011. PMID 21193625. PMC PMC3006050. Research Blogging.
- ↑ National Diabetes Information Clearinghouse. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). (2007) National Diabetes Statistics, 2007.
- ↑ Abdominal obesity-metabolic syndrome. (Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 605552. World Wide Web URL: http://omim.org/.)
- ↑ Diabetes mellitus, noninsulin-dependent. (Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 125853. World Wide Web URL: http://omim.org/.)
- ↑ Maturity-onset Diabetes of the Young. (Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 606391. World Wide Web URL: http://omim.org/.)
- ↑ Glaser B (January 2003). "Dominant SUR1 mutation causing autosomal dominant type 2 diabetes". Lancet 361 (9354): 272–3. DOI:10.1016/S0140-6736(03)12363-X. PMID 12559857. Research Blogging.
- ↑ Jump up to: 10.0 10.1 10.2 .World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus. Retrieved on 2007-05-29.
- ↑ Manley SE, Sikaris KA, Lu ZX, et al (February 2009). "Validation of an algorithm combining haemoglobin A(1c) and fasting plasma glucose for diagnosis of diabetes mellitus in UK and Australian populations". Diabet. Med. 26 (2): 115–21. DOI:10.1111/j.1464-5491.2008.02652.x. PMID 19236612. Research Blogging.
- ↑ Lu ZX, Walker KZ, O'Dea K, Sikaris KA, Shaw JE (2010). "A1C for screening and diagnosis of type 2 diabetes in routine clinical practice.". Diabetes Care 33 (4): 817-9. DOI:10.2337/dc09-1763. PMID 20067965. PMC PMC2845033. Research Blogging. Review in: Ann Intern Med. 2010 Aug 17;153(4):JC2-11
- ↑ (June 2009) "International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes". Diabetes Care. DOI:10.2337/dc09-9033. PMID 19502545. Research Blogging.
- ↑ Carson AP, Reynolds K, Fonseca VA, Muntner P (2010). "Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults.". Diabetes Care 33 (1): 95-7. DOI:10.2337/dc09-1227. PMID 19808920. PMC PMC2797994. Research Blogging.
- ↑ Jump up to: 15.0 15.1 (2005) "Diagnosis and classification of diabetes mellitus". Diabetes Care 28 Suppl 1: S37-42. PMID 15618111. [e]
- ↑ Kahn R (2010) Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis LancetDOI:10.1016/S0140-6736(09)62162-0
- ↑ U.S. Preventive Services Task Force (Sept 2014). "Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement". [e]
- ↑ U.S. Preventive Services Task Force (June 2008). "Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement". Ann. Intern. Med. 148 (11): 846–54. PMID 18519930. [e]
- ↑ Norris, Susan L.; Devan Kansagara, Christina Bougatsos, Rongwei Fu (2008-06-03). "Screening Adults for Type 2 Diabetes: A Review of the Evidence for the U.S. Preventive Services Task Force". Ann Intern Med 148 (11): 855-868. PMID 18519931. Retrieved on 2008-06-03.
- ↑ Hoerger TJ, Hicks KA, Sorensen SW, et al (2007). "Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. adults". Diabetes Care 30 (11): 2874–9. DOI:10.2337/dc07-0885. PMID 17698614. Research Blogging.
- ↑ U.S. Preventive Services Task Force (2003). "Screening for type 2 diabetes mellitus in adults: recommendations and rationale". Ann. Intern. Med. 138 (3): 212-4. PMID 12558361. National Guidelines Clearinghouse: Complete Summary
- ↑ Jump up to: 22.0 22.1 Harris R, Donahue K, Rathore SS, Frame P, Woolf SH, Lohr KN (2003). "Screening adults for type 2 diabetes: a review of the evidence for the U.S. Preventive Services Task Force". Ann. Intern. Med. 138 (3): 215-29. PMID 12558362.
- ↑ 10-year CVD Risk Calculator (Risk Assessment Tool for Estimating 10-year Risk of Developing Hard CHD (Myocardial Infarction and Coronary Death) Version). Retrieved on 2007-11-14.
- ↑ Care, Canadian Task Force on Preventive Health (2012-10-16). "Recommendations on screening for type 2 diabetes in adults". Canadian Medical Association Journal 184 (15): 1687-1696. DOI:10.1503/cmaj.120732. ISSN 1488-2329 0820-3946, 1488-2329. Retrieved on 2012-10-17. Research Blogging.
- ↑ Santaguida PL, Balion C, Hunt D, et al (2005). "Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose". Evidence report/technology assessment (Summary) (128): 1-11. PMID 16194123. [e]
- ↑ July 23, 2008 Diabetes Experts Recommend One-Two Punch for Treating Patients with Pre-Diabetes. American College of Endocrinology
- ↑ (2005) "Strategies to identify adults at high risk for type 2 diabetes: the Diabetes Prevention Program". Diabetes Care 28 (1): 138–44. PMID 15616247. [e]
- ↑ Icks A, Haastert B, Gandjour A, et al (2004). "Cost-effectiveness analysis of different screening procedures for type 2 diabetes: the KORA Survey 2000". Diabetes Care 27 (9): 2120–8. PMID 15333472. [e]
- ↑ Jump up to: 29.0 29.1 Heikes KE, Eddy DM, Arondekar B, Schlessinger L (May 2008). "Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes". Diabetes Care 31 (5): 1040–5. DOI:10.2337/dc07-1150. PMID 18070993. Research Blogging.
- ↑ Jump up to: 30.0 30.1 Collins GS, Altman DG (2011). "External validation of QDSCORE(®) for predicting the 10-year risk of developing Type 2 diabetes.". Diabet Med 28 (5): 599-607. DOI:10.1111/j.1464-5491.2011.03237.x. PMID 21480970. Research Blogging.
- ↑ Jump up to: 31.0 31.1 Ziemer DC, Kolm P, Foster JK, et al (2008). "Random Plasma Glucose in Serendipitous Screening for Glucose Intolerance: Screening for Impaired Glucose Tolerance Study 2". J Gen Intern Med. DOI:10.1007/s11606-008-0524-1. PMID 18335280. Research Blogging.
- ↑ Jump up to: 32.0 32.1 Rolka DB, Narayan KM, Thompson TJ, et al (2001). "Performance of recommended screening tests for undiagnosed diabetes and dysglycemia". Diabetes Care 24 (11): 1899-903. PMID 11679454. [e]
- ↑ Choi SH, Kim TH, Lim S, Park KS, Jang HC, Cho NH (2011). "Hemoglobin A1c as a Diagnostic Tool for Diabetes Screening and New-Onset Diabetes Prediction: A 6-year community-based prospective study.". Diabetes Care 34 (4): 944-9. DOI:10.2337/dc10-0644. PMID 21335372. PMC PMC3064055. Research Blogging.
- ↑ Pradhan AD, Rifai N, Buring JE, Ridker PM (2007). "Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women". Am. J. Med. 120 (8): 720-7. DOI:10.1016/j.amjmed.2007.03.022. PMID 17679132. Research Blogging.
- ↑ Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS (2010). "Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria.". Diabetes Care 33 (10): 2184-9. DOI:10.2337/dc10-0433. PMID 20639452. PMC PMC2945158. Research Blogging.
- ↑ Jump up to: 36.0 36.1 Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbæk A et al. (2011). "Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial.". Lancet 378 (9786): 156-67. DOI:10.1016/S0140-6736(11)60698-3. PMID 21705063. Research Blogging.
- ↑ Jump up to: 37.0 37.1 The NAVIGATOR Study Group (2010). "Effect of Valsartan on the Incidence of Diabetes and Cardiovascular Events.". N Engl J Med. DOI:10.1056/NEJMoa1001121. PMID 20228403. Research Blogging.
- ↑ Jump up to: 38.0 38.1 The NAVIGATOR Study Group (2010). "Effect of Nateglinide on the Incidence of Diabetes and Cardiovascular Events.". N Engl J Med. DOI:10.1056/NEJMoa1001122. PMID 20228402. Research Blogging.
- ↑ Jump up to: 39.0 39.1 39.2 Tuomilehto J, Lindström J, Eriksson JG, et al. (May 2001). "Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance". N. Engl. J. Med. 344 (18): 1343–50. PMID 11333990. [e]
- ↑ Jump up to: 40.0 40.1 40.2 Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J (2006). "Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study". Lancet 368 (9548): 1673-9. DOI:10.1016/S0140-6736(06)69701-8. PMID 17098085. Research Blogging. ACP Journal Club review
- ↑ Jump up to: 41.0 41.1 41.2 41.3 Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM (2002). "Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin". N. Engl. J. Med. 346 (6): 393-403. DOI:10.1056/NEJMoa012512. PMID 11832527. Research Blogging. ACP Journal Club review
- ↑ Jump up to: 42.0 42.1 42.2 Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S et al. (2005). "Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program.". Diabetes Care 28 (4): 888-94. PMID 15793191. PMC PMC1307521.
- ↑ Jump up to: 43.0 43.1 Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M (2003). "Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial". JAMA 290 (4): 486-94. DOI:10.1001/jama.290.4.486. PMID 12876091. Research Blogging. ACP Journal Club review
- ↑ Jump up to: 44.0 44.1 44.2 Gerstein HC, Yusuf S, Bosch J, et al (2006). "Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial". Lancet 368 (9541): 1096–105. DOI:10.1016/S0140-6736(06)69420-8. PMID 16997664. Research Blogging.
- ↑ Jump up to: 45.0 45.1 Salpeter SR, Buckley NS, Kahn JA, Salpeter EE (2008). "Meta-analysis: metformin treatment in persons at risk for diabetes mellitus". Am. J. Med. 121 (2): 149–157.e2. DOI:10.1016/j.amjmed.2007.09.016. PMID 18261504. Research Blogging.
- ↑ Jump up to: 46.0 46.1 Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K (May 2009). "Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance". Lancet 373 (9675): 1607–14. DOI:10.1016/S0140-6736(09)60222-1. PMID 19395079. Research Blogging.
- ↑ (2009)10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. LancetDOI:10.1016/S0140-6736(09)61457-4
- ↑ Gerstein HC, Yusuf S, Holman R, Bosch J, Pogue J (2004). "Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trial". Diabetologia 47 (9): 1519–27. DOI:10.1007/s00125-004-1485-5. PMID 15322749. Research Blogging.
- ↑ Eriksson KF, Lindgärde F (December 1991). "Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmö feasibility study". Diabetologia 34 (12): 891–8. DOI:10.1007/BF00400196. PMID 1778354. Research Blogging.
- ↑ Weng J, Li Y, Xu W, et al (May 2008). "Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial". Lancet 371 (9626): 1753–60. DOI:10.1016/S0140-6736(08)60762-X. PMID 18502299. Research Blogging. ACP Journal club summary
- ↑ Li Y, Xu W, Liao Z, et al (November 2004). "Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function". Diabetes Care 27 (11): 2597–602. PMID 15504992. [e]
- ↑ Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E (September 1997). "Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment". Diabetes Care 20 (9): 1353–6. PMID 9283777. [e]
- ↑ Park S, Choi SB (2003). "Induction of long-term normoglycemia without medication in Korean type 2 diabetes patients after continuous subcutaneous insulin infusion therapy". Diabetes Metab. Res. Rev. 19 (2): 124–30. DOI:10.1002/dmrr.343. PMID 12673780. Research Blogging.
- ↑ Ryan EA, Imes S, Wallace C (May 2004). "Short-term intensive insulin therapy in newly diagnosed type 2 diabetes". Diabetes Care 27 (5): 1028–32. PMID 15111515. [e]
- ↑ Retnakaran R, Drucker DJ (May 2008). "Intensive insulin therapy in newly diagnosed type 2 diabetes". Lancet 371 (9626): 1725–6. DOI:10.1016/S0140-6736(08)60736-9. PMID 18502278. Research Blogging.
- ↑ Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B et al. (2011). "Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials.". BMJ 343: d4169. DOI:10.1136/bmj.d4169. PMID 21791495. Research Blogging.
- ↑ Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO (2008). "The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis.". Ann Intern Med 149 (1): 11-9. PMID 18591633. PMC PMC2562733.
- ↑ Yau CK, Eng C, Cenzer IS, John Boscardin W, Rice-Trumble K, Lee SJ (2012). "Glycosylated hemoglobin and functional decline in community-dwelling nursing home-eligible elderly adults with diabetes mellitus.". J Am Geriatr Soc 60 (7): 1215-21. DOI:10.1111/j.1532-5415.2012.04041.x. PMID 22702660. Research Blogging.
- ↑ Jump up to: 59.0 59.1 59.2 Nathan DM, Buse JB, Davidson MB, et al (January 2009). "Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes". Diabetes Care 32 (1): 193–203. DOI:10.2337/dc08-9025. PMID 18945920. Research Blogging.
- ↑ Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK et al. (2007). "Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians.". Ann Intern Med 147 (6): 417-22. PMID 17876024. [e]
- ↑ Jump up to: 61.0 61.1 61.2 American Diabetes Association (January 2008). "Standards of medical care in diabetes--2008". Diabetes Care 31 Suppl 1: S12–54. DOI:10.2337/dc08-S012. PMID 18165335. Research Blogging. Complete summary from National Guidelines Clearinghouse
- ↑ (January 2006) "Standards of medical care in diabetes--2006". Diabetes Care 29 Suppl 1: S4–42. PMID 16373931. [e]
- ↑ Woo V (March 2009). "Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes: response to Nathan et al". Diabetes Care 32 (3): e34; author reply e37–8. DOI:10.2337/dc08-2093. PMID 19246585. Research Blogging.
- ↑ Home P, Mant J, Diaz J, Turner C (June 2008). "Management of type 2 diabetes: summary of updated NICE guidance". BMJ 336 (7656): 1306–8. DOI:10.1136/bmj.39560.442095.AD. PMID 18535074. PMC 2413390. Research Blogging.
- ↑ NICE guidance by type. Retrieved on 2008-08-26.
- ↑ Anonymous. NICE guidelines for diabetes mellitus, type 2. Daily POEMS. Essential Evidence Plus. Retrieved on 2008-08-26.
- ↑ Brown AF, Mangione CM, Saliba D, Sarkisian CA (2003). "Guidelines for improving the care of the older person with diabetes mellitus". Journal of the American Geriatrics Society 51 (5 Suppl Guidelines): S265–80. DOI:10.1046/j.1532-5415.51.5s.1.x. PMID 12694461. Research Blogging.
- ↑ (1998) "Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group". Lancet 352 (9131): 837–53. DOI:10.1016/S0140-6736(98)07019-6. PMID 9742976. Research Blogging. Review by ACP Journal Club
- ↑ (1998) "Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group.". Lancet 352 (9131): 854-65. PMID 9742977.
- ↑ Duckworth W, Abraira C, Moritz T, et al (January 2009). "Glucose control and vascular complications in veterans with type 2 diabetes". N. Engl. J. Med. 360 (2): 129–39. DOI:10.1056/NEJMoa0808431. PMID 19092145. Research Blogging.
- ↑ Abraira C, Duckworth W, McCarren M, et al (2003). "Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial". Journal of diabetes and its complications 17 (6): 314–22. PMID 14583175. [e]
- ↑ (June 2008) "Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes". N. Engl. J. Med. 358 (24): 2560–2572. DOI:10.1056/NEJMoa0802987. PMID 18539916. Research Blogging.
- ↑ Anonymous (February 6, 2008). For Safety, NHLBI Changes Intensive Blood Sugar Treatment Strategy in Clinical Trial of Diabetes and Cardiovascular Disease -. National Institutes of Health (NIH). Retrieved on 2008-02-07.
- ↑ Gerstein HC, Miller ME, Byington RP, et al (June 2008). "Effects of intensive glucose lowering in type 2 diabetes". N. Engl. J. Med. 358 (24): 2545–59. DOI:10.1056/NEJMoa0802743. PMID 18539917. Research Blogging.
- ↑ The ORIGIN Trial Investigators (2012). "Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia.". N Engl J Med. DOI:10.1056/NEJMoa1203858. PMID 22686416. Research Blogging.
- ↑ Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK et al. (2005). "Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial.". Lancet 366 (9493): 1279-89. DOI:10.1016/S0140-6736(05)67528-9. PMID 16214598. Research Blogging. Review in: ACP J Club. 2006 Mar-Apr;144(2):34 Review in: Evid Based Med. 2006 Apr;11(2):47
- ↑ Meinert CL, Knatterud GL, Prout TE, Klimt CR (1970). "A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results". Diabetes 19: Suppl:789–830. PMID 4926376. [e]
- ↑ (1982) "Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VIII. Evaluation of insulin therapy: final report.". Diabetes 31 Suppl 5: 1-81. PMID 6757026. [e]
- ↑ Jump up to: 79.0 79.1 Kilo C, Miller JP, Williamson JR (1980). "The Achilles heel of the University Group Diabetes Program.". JAMA 243 (5): 450-7. DOI:10.1001/jama.1980.03300310038020. PMID 6985989. Research Blogging.
- ↑ Jump up to: 80.0 80.1 80.2 Genuth S (1996). "Exogenous insulin administration and cardiovascular risk in non-insulin-dependent and insulin-dependent diabetes mellitus.". Ann Intern Med 124 (1 Pt 2): 104-9. PMID 8554200.
- ↑ Feinglos MN, Bethel MA (1999). "Therapy of type 2 diabetes, cardiovascular death, and the UGDP.". Am Heart J 138 (5 Pt 1): S346-52. DOI:10.1016/S0002-8703(99)70034-7. PMID 10539796. Research Blogging.
- ↑ Gaster B, Hirsch IB (1998). "The effects of improved glycemic control on complications in type 2 diabetes.". Arch Intern Med 158 (2): 134-40. PMID 9448551.
- ↑ Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM et al. (2012). "Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline.". J Clin Endocrinol Metab 97 (1): 16-38. DOI:10.1210/jc.2011-2098. PMID 22223765. Research Blogging.
- ↑ Qaseem, Amir; Linda L. Humphrey, Roger Chou, Vincenza Snow, Paul Shekelle, for the Clinical Guidelines Committee of the American College of Physicians (2011-02-15). "Use of Intensive Insulin Therapy for the Management of Glycemic Control in Hospitalized Patients: A Clinical Practice Guideline From the American College of Physicians". Annals of Internal Medicine 154 (4): 260 -267. DOI:10.1059/0003-4819-154-4-201102150-00007. Retrieved on 2011-02-15. Research Blogging.
- ↑ Qaseem A, Chou R, Humphrey LL, Shekelle P, for the Clinical Guidelines Committee of the American College of Physicians (2013). "Inpatient Glycemic Control: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians.". Am J Med Qual. DOI:10.1177/1062860613489339. PMID 23709472. Research Blogging.
- ↑ Jump up to: 86.0 86.1 Mulrow CD, Lohr KN (April 2001). "Proof and policy from medical research evidence". J Health Polit Policy Law 26 (2): 249–66. PMID 11330080. [e]
- ↑ Jump up to: 87.0 87.1 AACE Diabetes Mellitus Clinical Practice Guidelines Task Force (2007). "American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus". Endocr Pract 13 Suppl 1: 1–68. PMID 17613449. [e] Complete summary from National Guidelines Clearinghouse
- ↑ Soylemez Wiener R, Wiener DC, Larson RJ (August 2008). "Benefits and risks of tight glucose control in critically ill adults: a meta-analysis". JAMA 300 (8): 933–44. DOI:10.1001/jama.300.8.933. PMID 18728267. Research Blogging.
- ↑ The NICE-SUGAR Study Investigators (2009-03-24). "Intensive versus Conventional Glucose Control in Critically Ill Patients". N Engl J Med: NEJMoa0810625. DOI:10.1056/NEJMoa0810625. Retrieved on 2009-03-24. Research Blogging.
- ↑ Arabi YM, Dabbagh OC, Tamim HM, et al (December 2008). "Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients". Crit. Care Med. 36 (12): 3190–7. DOI:10.1097/CCM.0b013e31818f21aa. PMID 18936702. Research Blogging.
- ↑ http://pubmed.gov/22090269
- ↑ Gandhi GY, Nuttall GA, Abel MD, et al (2007). "Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial". Ann. Intern. Med. 146 (4): 233–43. PMID 17310047. [e]
- ↑ Vlasselaers et al. (2009) Lancet. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. DOI:10.1016/S0140-6736(09)60044-1
- ↑ Farmer AJ, Perera R, Ward A, Heneghan C, Oke J, Barnett AH et al. (2012). "Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes.". BMJ 344: e486. DOI:10.1136/bmj.e486. PMID 22371867. Research Blogging. Review in: Ann Intern Med. 2012 Jun 19;156(12):JC6-12
- ↑ Farmer A, Wade A, Goyder E, et al (2007). "Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial". DOI:10.1136/bmj.39247.447431.BE. PMID 17591623. Research Blogging.
- ↑ O'Kane, M. J., Bunting, B., Copeland, M., Coates, V. E., & on behalf of the ESMON study group. (2008). Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ, bmj.39534.571644.BE. DOI:10.1136/bmj.39534.571644.BE
- ↑ Jenkins, David J. A.; Cyril W. C. Kendall, Gail McKeown-Eyssen, Robert G. Josse, Jay Silverberg, Gillian L. Booth, Edward Vidgen, Andrea R. Josse, Tri H. Nguyen, Sorcha Corrigan, Monica S. Banach, Sophie Ares, Sandy Mitchell, Azadeh Emam, Livia S. A. Augustin, Tina L. Parker, Lawrence A. Leiter (2008-12-17). "Effect of a Low-Glycemic Index or a High-Cereal Fiber Diet on Type 2 Diabetes: A Randomized Trial". JAMA 300 (23): 2742-2753. DOI:10.1001/jama.2008.808. Retrieved on 2008-12-17. Research Blogging.
- ↑ Jump up to: 98.0 98.1 Phung OJ, Scholle JM, Talwar M, Coleman CI (2010). "Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes.". JAMA 303 (14): 1410-8. DOI:10.1001/jama.2010.405. PMID 20388897. Research Blogging.
- ↑ Loke, Y. K.; C. S. Kwok, S. Singh (2011). "Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies". BMJ 342 (mar17 1): d1309-d1309. DOI:10.1136/bmj.d1309. ISSN 0959-8138. Retrieved on 2011-03-24. Research Blogging.
- ↑ Amori RE, Lau J, Pittas AG (2007). "Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis.". JAMA 298 (2): 194-206. DOI:10.1001/jama.298.2.194. PMID 17622601. Research Blogging. Review in: ACP J Club. 2008 Jan-Feb;148(1):3
- ↑ Jump up to: 101.0 101.1 Parks M, Rosebraugh C (2010). "Weighing risks and benefits of liraglutide--the FDA's review of a new antidiabetic therapy.". N Engl J Med 362 (9): 774-7. DOI:10.1056/NEJMp1001578. PMID 20164475. Research Blogging.
- ↑ Masur K, Schwartz F, Entschladen F, Niggemann B, Zaenker KS (2006). "DPPIV inhibitors extend GLP-2 mediated tumour promoting effects on intestinal cancer cells.". Regul Pept 137 (3): 147-55. DOI:10.1016/j.regpep.2006.07.003. PMID 16908079. Research Blogging.
- ↑ Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K et al. (2010). "Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial.". Lancet 375 (9733): 2234-43. DOI:10.1016/S0140-6736(10)60406-0. PMID 20609969. Research Blogging.
- ↑ Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S et al. (2010). "Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial.". Lancet 375 (9724): 1447-56. DOI:10.1016/S0140-6736(10)60307-8. PMID 20417856. Research Blogging.
- ↑ Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL (2008). "Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus.". Cochrane Database Syst Rev (2): CD006739. DOI:10.1002/14651858.CD006739.pub2. PMID 18425967. Research Blogging.
- ↑ Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 008 Group (2009). "Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study.". Int J Clin Pract 63 (1): 46-55. DOI:10.1111/j.1742-1241.2008.01933.x. PMID 19125992. Research Blogging.
- ↑ Bolen S et al. Systematic Review: Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus. Ann Intern Med 2007;147:6
- ↑ Kahn SE, Haffner SM, Heise MA, et al (2006). "Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy". N. Engl. J. Med. 355 (23): 2427-43. DOI:10.1056/NEJMoa066224. PMID 17145742. Research Blogging.
- ↑ Eurich DT, McAlister FA, Blackburn DF, et al (2007). "Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review". BMJ 335 (7618): 497. DOI:10.1136/bmj.39314.620174.80. PMID 17761999. Research Blogging.
- ↑ Jump up to: 110.0 110.1 Mooradian AD, Bernbaum M, Albert SG (2006). "Narrative review: a rational approach to starting insulin therapy". Ann. Intern. Med. 145 (2): 125-34. PMID 16847295. [e]
- ↑ Jump up to: 111.0 111.1 111.2 111.3 Yki-Järvinen H, Kauppila M, Kujansuu E, et al (1992). "Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus". N. Engl. J. Med. 327 (20): 1426-33. PMID 1406860. [e]
- ↑ Jump up to: 112.0 112.1 Yki-Järvinen H, Ryysy L, Nikkilä K, Tulokas T, Vanamo R, Heikkilä M (1999). "Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial". Ann. Intern. Med. 130 (5): 389–96. PMID 10068412. [e]
- ↑ Kratz A, Lewandrowski KB (1998). "Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Normal reference laboratory values". N. Engl. J. Med. 339 (15): 1063–72. PMID 9761809. [e]
- ↑ Holman RR, Turner RC (1985). "A practical guide to basal and prandial insulin therapy". Diabet. Med. 2 (1): 45–53. PMID 2951066. [e]
- ↑ Jump up to: 115.0 115.1 Holman RR, Thorne KI, Farmer AJ, et al (2007). "Addition of Biphasic, Prandial, or Basal Insulin to Oral Therapy in Type 2 Diabetes". N. Engl. J. Med. 357. DOI:10.1056/NEJMoa075392. PMID 17890232. Research Blogging.
- ↑ Jump up to: 116.0 116.1 Raskin P, Allen E, Hollander P, et al (2005). "Initiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogs". Diabetes Care 28 (2): 260-5. PMID 15677776. [e]
- ↑ Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH (2004). "Combined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy". Clinical therapeutics 26 (12): 2034-44. DOI:10.1016/j.clinthera.2004.12.015. PMID 15823767. Research Blogging.
- ↑ Horvath K, Jeitler K, Berghold A, Ebrahim Sh, Gratzer T, Plank J, Kaiser T, Pieber T, Siebenhofer A (2007). "Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus". Cochrane database of systematic reviews (Online) (2): CD005613. PMID 17443605.
- ↑ Esposito K, Ciotola M, Maiorino MI, et al. (October 2008). "Addition of neutral protamine lispro insulin or insulin glargine to oral type 2 diabetes regimens for patients with suboptimal glycemic control: a randomized trial". Ann. Intern. Med. 149 (8): 531–9. PMID 18936501. [e]
- ↑ Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF et al. (2009). "Three-year efficacy of complex insulin regimens in type 2 diabetes.". N Engl J Med 361 (18): 1736-47. DOI:10.1056/NEJMoa0905479. PMID 19850703. Research Blogging.
- ↑ Howard, B. V., Roman, M. J., Devereux, R. B., Fleg, J. L., Galloway, J. M., Henderson, J. A., et al. (2008). Effect of Lower Targets for Blood Pressure and LDL Cholesterol on Atherosclerosis in Diabetes: The SANDS Randomized Trial. JAMA, 299(14), 1678-1689. DOI:10.1001/jama.299.14.1678.
- ↑ Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N et al. (2008). "Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial.". JAMA 300 (18): 2134-41. DOI:10.1001/jama.2008.623. PMID 18997198. Research Blogging.
- ↑ Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (2000). "Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators". N. Engl. J. Med. 342 (3): 145-53. PMID 10639539. [e]
- ↑ Pilote L, Abrahamowicz M, Rodrigues E, Eisenberg MJ, Rahme E (2004). "Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction: a class effect?". Ann. Intern. Med. 141 (2): 102-12. PMID 15262665. [e]
- ↑ Svensson P, de Faire U, Sleight P, Yusuf S, Ostergren J (2001). "Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE Substudy.". Hypertension 38 (6): E28-32. PMID 11751742.
- ↑ Kurtz TW (2003). "False claims of blood pressure-independent protection by blockade of the renin angiotensin aldosterone system?". Hypertension 41 (2): 193-6. PMID 12574079.
- ↑ Jump up to: 127.0 127.1 127.2 The ACCORD Study Group (2010). "Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus.". N Engl J Med. DOI:10.1056/NEJMoa1001286. PMID 20228401. Research Blogging.
- ↑ Jump up to: 128.0 128.1 Patel A, ADVANCE Collaborative Group. MacMahon S, Chalmers J, Neal B, Woodward M et al. (2007). "Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial.". Lancet 370 (9590): 829-40. DOI:10.1016/S0140-6736(07)61303-8. PMID 17765963. Research Blogging. Review in: Evid Based Med. 2008 Apr;13(2):49
- ↑ Chobanian AV, Bakris GL, Black HR, et al (2003). "The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report". JAMA 289 (19): 2560-72. DOI:10.1001/jama.289.19.2560. PMID 12748199. Research Blogging.
- ↑ Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V et al. (2004). "Preventing microalbuminuria in type 2 diabetes.". N Engl J Med 351 (19): 1941-51. DOI:10.1056/NEJMoa042167. PMID 15516697. Research Blogging. Review in: ACP J Club. 2005 May-Jun;142(3):65
- ↑ Snow V, Aronson M, Hornbake E, Mottur-Pilson C, Weiss K (2004). "Lipid control in the management of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians". Ann Intern Med 140 (8): 644-9. PMID 15096336.
- ↑ Jump up to: 132.0 132.1 Vijan S, Hayward RA, American College of Physicians (2004). "Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American College of Physicians.". Ann Intern Med 140 (8): 650-8. PMID 15096337. [e] Review in: ACP J Club. 2004 Nov-Dec;141(3):65
- ↑ Kearney PM, Blackwell L, Collins R, et al (2008). "Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis". Lancet 371 (9607): 117–25. DOI:10.1016/S0140-6736(08)60104-X. PMID 18191683. Research Blogging.
- ↑ Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I (February 2009). "Is diabetes a coronary risk equivalent? Systematic review and meta-analysis". Diabet. Med. 26 (2): 142–8. DOI:10.1111/j.1464-5491.2008.02640.x. PMID 19236616. Research Blogging.
- ↑ Howard, B. V., Roman, M. J., Devereux, R. B., Fleg, J. L., Galloway, J. M., Henderson, J. A., et al. (2008). Effect of Lower Targets for Blood Pressure and LDL Cholesterol on Atherosclerosis in Diabetes: The SANDS Randomized Trial. JAMA, 299(14), 1678-1689. DOI:10.1001/jama.299.14.1678.
- ↑ John B. Dixon et al., “Adjustable Gastric Banding and Conventional Therapy for Type 2 Diabetes: A Randomized Controlled Trial,” JAMA 299, no. 3 (January 23, 2008).
- ↑ Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L et al. (2010). "Severe hypoglycemia and risks of vascular events and death.". N Engl J Med 363 (15): 1410-8. DOI:10.1056/NEJMoa1003795. PMID 20925543. Research Blogging.