User:Milton Beychok/Sandbox: Difference between revisions
Jump to navigation
Jump to search
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 45: | Line 45: | ||
|- align="center" | |- align="center" | ||
|[[Water]]||H<sub>2</sub>0||colspan=2|40,660||100||373.2||colspan=2|647.3||colspan=2|218.3 | |[[Water]]||H<sub>2</sub>0||colspan=2|40,660||100||373.2||colspan=2|647.3||colspan=2|218.3 | ||
|- | |||
|colspan=10|:(1)H<sub>v</sub> is the heat of vaporization<br /> | |||
:(2) T<sub>n</sub> = normal boiling point<br /> | |||
:(3) T<sub>c</sub> = critical temperature<br/> | |||
:(4) P<sub>c</sub> = absolute critical pressure | |||
|} | |} | ||
Revision as of 01:08, 8 September 2009
| Name | Formula | Hv | Tn | Tc | Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| ( J/mol ) | ( °C ) | ( K ) | ( K ) | ( atm ) | |||||
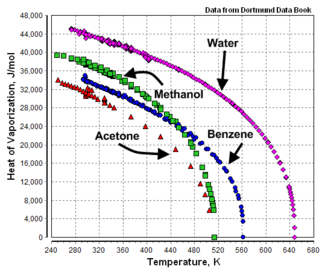
| Acetic acid | C2H4O2 | 23,700 | 117.9 | 391.1 | 594.8 | 57.1 | |||
| Acetone | C3H6O | 29,100 | 56.2 | 329.4 | 508.7 | 47.0 | |||
| Benzene | C6H6 | 30,720 | 80.0 | 353.2 | 562.1 | 48.6 | |||
| Butane | C4H10 | 22,440 | – 0.5 | 272.7 | 425.2 | 37.5 | |||
| Carbon tetrachloride | CCl4 | 29,820 | 76.6 | 349.8 | 556.3 | 45.0 | |||
| Chloroform | CHCl3 | 29,240 | 61.1 | 334.3 | 536.2 | 54.0 | |||
| Cyclohexane | C6H12 | 29,970 | 80.7 | 353.9 | 553.6 | 40.0 | |||
| Cyclopentane | C5H10 | 27,300 | 49.2 | 322.4 | 511.8 | 44.6 | |||
| Decane | C10H22 | 38,750 | 174.1 | 447.3 | 617.6 | 20.8 | |||
| Ethanol | C2H6O | 38,560 | 78.2 | 351.4 | 516.2 | 63.0 | |||
| Hexane | C6H14 | 28.850 | 68.7 | 341.9 | 507.4 | 29.9 | |||
| Isobutane | C4H10 | 21,300 | – 11.9 | 261.3 | 408.2 | 36.0 | |||
| Methanol | CH4O | 35,210 | 64.7 | 337.9 | 513.2 | 78.5 | |||
| Octane | C8H18 | 34,410 | 125.6 | 398.8 | 569.2 | 24.8 | |||
| Water | H20 | 40,660 | 100 | 373.2 | 647.3 | 218.3 | |||
:(1)Hv is the heat of vaporization
| |||||||||
References
- ↑ Dortmund Data Bank Online Search
- ↑ J.M. Smith, H.C. Van Ness and N.M. Abbot (2004). Introduction to Chemical Engineering Thermodynamics, 7th Edition. McGraw-Hill. ISBN 0-07-310445-0.
- ↑ Robert C. Weast (Editor) (1976). Perry's Chemical Engineers' Handbook, 56th Edition. CRC Press. ISBN 0-87819-455-X.