User:Milton Beychok/Sandbox: Difference between revisions
Jump to navigation
Jump to search
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
{{TOC|right}} | |||
The '''heat of vaporization''', (<math>H_v</math>) is the amount of thermal [[energy] required to convert a quantity of [[liquid]] into a [[vapor]]. It can be thought of as the energy required to break the | |||
intermolecular bonds within the liquid. | |||
It is also often referred to as the '''latent heat of vaporization''' (s<math>LH_v</math> or <math>L_v</math>) and the '''enthalpy of vaporization''' (symbol <math>H_v</math> or <math>\Delta H_v</math>) and is usually measured and reported at the [[temperature]] corresponding to the [[normal boiling point]] of the liquid. | |||
== Units == | |||
Values are usually quoted in [[Joule|J]]/[[Mole (unit)|mol]] or kJ/mol (molar heat of vaporization), although kJ/[[Kilogram|kg]] or J/g (specific heat of vaporization), and older units like [[Calorie|kcal]]/mol, cal/g and [[British thermal unit|Btu]]/[[Pound (mass)|lb]] are sometimes still used, among others. | |||
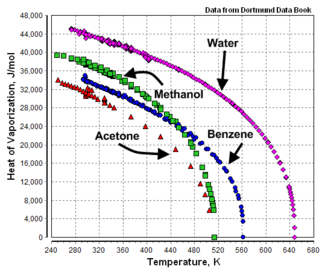
{{Image|Heat of Vaporization.png|right|325px|Heats of vaporization versus temperature.<ref>[http://www.ddbst.com/new/Default.htm Dortmund Data Bank Online Search]</ref>}} | {{Image|Heat of Vaporization.png|right|325px|Heats of vaporization versus temperature.<ref>[http://www.ddbst.com/new/Default.htm Dortmund Data Bank Online Search]</ref>}} | ||
Revision as of 16:45, 8 September 2009
The heat of vaporization, () is the amount of thermal [[energy] required to convert a quantity of liquid into a vapor. It can be thought of as the energy required to break the intermolecular bonds within the liquid.
It is also often referred to as the latent heat of vaporization (s or ) and the enthalpy of vaporization (symbol or ) and is usually measured and reported at the temperature corresponding to the normal boiling point of the liquid.
Units
Values are usually quoted in J/mol or kJ/mol (molar heat of vaporization), although kJ/kg or J/g (specific heat of vaporization), and older units like kcal/mol, cal/g and Btu/lb are sometimes still used, among others.
| Name | Formula | Hv | Tn | Tc | Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| ( J/mol ) | ( °C ) | ( K ) | ( K ) | ( atm ) | |||||
| Acetic acid | C2H4O2 | 23,700 | 117.9 | 391.1 | 594.8 | 57.1 | |||
| Acetone | C3H6O | 29,100 | 56.2 | 329.4 | 508.7 | 47.0 | |||
| Benzene | C6H6 | 30,720 | 80.0 | 353.2 | 562.1 | 48.6 | |||
| Butane | C4H10 | 22,440 | – 0.5 | 272.7 | 425.2 | 37.5 | |||
| Carbon tetrachloride | CCl4 | 29,820 | 76.6 | 349.8 | 556.3 | 45.0 | |||
| Chloroform | CHCl3 | 29,240 | 61.1 | 334.3 | 536.2 | 54.0 | |||
| Cyclohexane | C6H12 | 29,970 | 80.7 | 353.9 | 553.6 | 40.0 | |||
| Cyclopentane | C5H10 | 27,300 | 49.2 | 322.4 | 511.8 | 44.6 | |||
| Decane | C10H22 | 38,750 | 174.1 | 447.3 | 617.6 | 20.8 | |||
| Ethanol | C2H6O | 38,560 | 78.2 | 351.4 | 516.2 | 63.0 | |||
| Hexane | C6H14 | 28.850 | 68.7 | 341.9 | 507.4 | 29.9 | |||
| Isobutane | C4H10 | 21,300 | – 11.9 | 261.3 | 408.2 | 36.0 | |||
| Methanol | CH4O | 35,210 | 64.7 | 337.9 | 513.2 | 78.5 | |||
| Octane | C8H18 | 34,410 | 125.6 | 398.8 | 569.2 | 24.8 | |||
| Water | H20 | 40,660 | 100 | 373.2 | 647.3 | 218.3 | |||
Notes:
| |||||||||
References
- ↑ Dortmund Data Bank Online Search
- ↑ J.M. Smith, H.C. Van Ness and N.M. Abbot (2004). Introduction to Chemical Engineering Thermodynamics, 7th Edition. McGraw-Hill. ISBN 0-07-310445-0.
- ↑ Robert C. Weast (Editor) (1976). Perry's Chemical Engineers' Handbook, 56th Edition. CRC Press. ISBN 0-87819-455-X.