User:Milton Beychok/Sandbox: Difference between revisions
Jump to navigation
Jump to search
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 3: | Line 3: | ||
{| class = "wikitable" align="left" | {| class = "wikitable" align="left" | ||
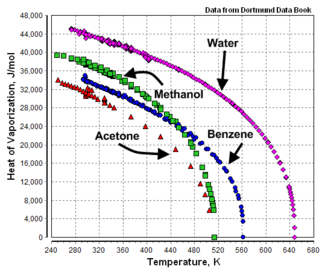
|+ Heat of vaporization, normal boiling point, and critical<br/>points of various liquids | |+ Heat of vaporization, normal boiling point, and critical<br/>points of various liquids | ||
!Name! | |- | ||
|- | !rowspan=2|Name | ||
!rowspan=2|Formula | |||
!rowspan=2|H<sub>v</sub><br/>( J/mol )!!colspan=2|T<sub>n</sub>!!rowspan=2|T<sub>c</sub><br/>( K )!!rowspan=2 |P<sub>c</sub><br/>( atm ) | |||
|- | |||
!( °C ) | |||
!( K ) | |||
|} | |} | ||