User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
'''Petroleum crude oil''' is a naturally occurring, [[flammability|flammable]] liquid found primarily in underground rock formations and consists of a complex mixture of [[hydrocarbon]]s of various [[molecular weight]]s plus other [[organic compound]]s. | '''Petroleum crude oil''', or simply '''crude oil''', is a naturally occurring, [[flammability|flammable]] liquid found primarily in underground rock formations and consists of a complex mixture of [[hydrocarbon]]s of various [[molecular weight]]s plus other [[organic compound]]s. | ||
The [[Latin]] word '''''petroleum''''' was first used to describe petroleum crude oil by the [[Germany|German]] mineralogist [[Georg Bauer]] (also known as Georgius Agricola) in the treatise ''De Natura Fossilium'', published in 1546<ref>{{cite book |author=Bauer Georg|title=De Natura Fossilium |year=1546}} Translated in 1955 by Mark C. Bandy and Jean A. Bandy </ref> The [[Greek language|Greek]] word for petroleum is ''πετρέλαιον'', meaning "rock oil". | The [[Latin]] word '''''petroleum''''' was first used to describe petroleum crude oil by the [[Germany|German]] mineralogist [[Georg Bauer]] (also known as Georgius Agricola) in the treatise ''De Natura Fossilium'', published in 1546<ref>{{cite book |author=Bauer Georg|title=De Natura Fossilium |year=1546}} Translated in 1955 by Mark C. Bandy and Jean A. Bandy </ref> The [[Greek language|Greek]] word for petroleum is ''πετρέλαιον'', meaning "rock oil". | ||
== Composition == | == Composition of crude oil == | ||
Both crude oil and [[natural gas]] are predominantly mixtures of [[hydrocarbons]]. At typical ambient conditions of pressure and temperature, the lower molecular weight hydrocarbons [[methane]], [[ethane]], [[propane]] and [[butane]] occur as gases, while the higher molecular weight ones ([[pentane]] and higher) are in the form of liquids or solids. However, in the underground [[oil reservoir]]s the proportion which is gas or liquid varies depending on the subsurface conditions, and on the [[phase diagram]] of the petroleum mixture.<ref name="Hyne 2001">{{cite book|author=Norman J. Hyne|title=Nontechnical Guide to Petroleum Geology, Exploration, Drilling, and Production|edition=|publisher=PennWell Corporation|date=2001|pages=pages 1-4|id=ISBN 087814823X}}</ref> | Both crude oil and [[natural gas]] are predominantly mixtures of [[hydrocarbons]]. At typical ambient conditions of pressure and temperature, the lower molecular weight hydrocarbons [[methane]], [[ethane]], [[propane]] and [[butane]] occur as gases, while the higher molecular weight ones ([[pentane]] and higher) are in the form of liquids or solids. However, in the underground [[oil reservoir]]s the proportion which is gas or liquid varies depending on the subsurface conditions, and on the [[phase diagram]] of the petroleum mixture.<ref name="Hyne 2001">{{cite book|author=Norman J. Hyne|title=Nontechnical Guide to Petroleum Geology, Exploration, Drilling, and Production|edition=|publisher=PennWell Corporation|date=2001|pages=pages 1-4|id=ISBN 087814823X}}</ref> | ||
Crude oil consists mostly of hydrocarbons with small amounts of other [[Organic chemistry|organic chemical compounds]] that may contain [[nitrogen]], [[oxygen]] or [[sulfur]]. It may also contain trace amounts of metals such as [[iron]], [[nickel]], [[copper]] and [[vanadium]]. The exact molecular composition varies widely from formation to formation but the proportion of [[chemical element]]s vary over fairly narrow limits as follows:<ref name="Speight"> {{Cite book | |||
| last = Speight | | last = Speight | ||
| first = James G. | | first = James G. | ||
| Line 40: | Line 36: | ||
|} | |} | ||
;The hydrocarbons in crude oil: | |||
{{main|Hydrocarbons|Petroleum refining processes|Petrochemical}} | {{main|Hydrocarbons|Petroleum refining processes|Petrochemical}} | ||
Petroleum is a mixture of a very large number of different [[hydrocarbon]]s. The most common hydrocarbons found in petroleum crude oil are linear or branched [[Hydrocarbons|alkane]]s (also called ''paraffins''), [[Hydrocarbons|cycloalkane]]s (also called ''cyclic paraffins'' or ''naphthenes''), [[Hydrocarbons|aromatic hydrocarbon]]s, or much more complicated chemicals like [[asphaltene]]s which may have a [[molecular weight]] of 800 to 500.<ref>{{cite book|author=Oliver Mullins and Eric Sheu (Editors)|title=Structure & Dynamics of Asphaltenes|edition=1st Edition|publisher=Springer|year=1999|id=ISBN 0-306-45930-2}} (See Chapter 1, page 17)</ref><ref>Note: There are many other values in the technical literature for the molecular weight of asphaltenes and there does not appear to be a concensus as to which values are more correct.</ref> . | Petroleum is a mixture of a very large number of different [[hydrocarbon]]s. The most common hydrocarbons found in petroleum crude oil are linear or branched [[Hydrocarbons|alkane]]s (also called ''paraffins''), [[Hydrocarbons|cycloalkane]]s (also called ''cyclic paraffins'' or ''naphthenes''), [[Hydrocarbons|aromatic hydrocarbon]]s, or much more complicated chemicals like [[asphaltene]]s which may have a [[molecular weight]] of 800 to 500.<ref>{{cite book|author=Oliver Mullins and Eric Sheu (Editors)|title=Structure & Dynamics of Asphaltenes|edition=1st Edition|publisher=Springer|year=1999|id=ISBN 0-306-45930-2}} (See Chapter 1, page 17)</ref><ref>Note: There are many other values in the technical literature for the molecular weight of asphaltenes and there does not appear to be a concensus as to which values are more correct.</ref> . | ||
| Line 135: | Line 83: | ||
Geologists often refer to the temperature range in which oil forms as an "oil window"—below the minimum temperature oil remains trapped in the form of kerogen, and above the maximum temperature the oil is converted to [[natural gas]] through the process of [[thermal cracking]]. Although this temperature range is found at different depths below the surface throughout the world, a typical depth for the oil window is 4–6 km. Sometimes, oil which is formed at extreme depths may migrate and become trapped at much shallower depths than where it was formed. The [[Athabasca Oil Sands]] is one example of this. | Geologists often refer to the temperature range in which oil forms as an "oil window"—below the minimum temperature oil remains trapped in the form of kerogen, and above the maximum temperature the oil is converted to [[natural gas]] through the process of [[thermal cracking]]. Although this temperature range is found at different depths below the surface throughout the world, a typical depth for the oil window is 4–6 km. Sometimes, oil which is formed at extreme depths may migrate and become trapped at much shallower depths than where it was formed. The [[Athabasca Oil Sands]] is one example of this. | ||
== Crude oil == | == Crude oil == | ||
| Line 159: | Line 105: | ||
==Classification== | ==Classification== | ||
The [[petroleum industry]] generally classifies crude oil by the geographic location of the reservoir from which it is | The [[petroleum industry]] generally classifies crude oil by the geographic location of the reservoir from which it is produced (e.g. [[West Texas Intermediate]], [[Brent oilfield|Brent]], or [[Oman]]), its [[API gravity]] (an oil industry measure of density), and by its sulfur content. Crude oil may be considered ''light'' if it has a low density or ''heavy'' if it has a high density and it may be referred to as ''sweet'' if it contains relatively little sulfur or ''sour'' if it contains substantial amounts of sulfur. | ||
''Light'' crude oil is more desirable than ''heavy'' oil since it provides a higher yield of gasoline and ''sweet'' oil is more desirable than ''sour'' oil because it has fewer environmental problems and requires less refining to meet sulfur content standards of refined fuels. Each crude oil has a unique composition and set of physical properties which are delineated by [[crude oil assay]]s performed in petroleum laboratories. | ''Light'' crude oil is more desirable than ''heavy'' oil since it provides a higher yield of gasoline and ''sweet'' oil is more desirable than ''sour'' oil because it has fewer environmental problems and requires less refining to meet sulfur content standards of refined fuels. Each crude oil has a unique composition and set of physical properties which are delineated by [[crude oil assay]]s performed in petroleum laboratories. | ||
Revision as of 02:48, 4 October 2009
Petroleum crude oil, or simply crude oil, is a naturally occurring, flammable liquid found primarily in underground rock formations and consists of a complex mixture of hydrocarbons of various molecular weights plus other organic compounds.
The Latin word petroleum was first used to describe petroleum crude oil by the German mineralogist Georg Bauer (also known as Georgius Agricola) in the treatise De Natura Fossilium, published in 1546[1] The Greek word for petroleum is πετρέλαιον, meaning "rock oil".
Composition of crude oil
Both crude oil and natural gas are predominantly mixtures of hydrocarbons. At typical ambient conditions of pressure and temperature, the lower molecular weight hydrocarbons methane, ethane, propane and butane occur as gases, while the higher molecular weight ones (pentane and higher) are in the form of liquids or solids. However, in the underground oil reservoirs the proportion which is gas or liquid varies depending on the subsurface conditions, and on the phase diagram of the petroleum mixture.[2]
Crude oil consists mostly of hydrocarbons with small amounts of other organic chemical compounds that may contain nitrogen, oxygen or sulfur. It may also contain trace amounts of metals such as iron, nickel, copper and vanadium. The exact molecular composition varies widely from formation to formation but the proportion of chemical elements vary over fairly narrow limits as follows:[3]
| Element | Percent range |
|---|---|
| Carbon | 83 to 87% |
| Hydrogen | 10 to 14% |
| Nitrogen | 0.1 to 2% |
| Oxygen | 0.1 to 1.5% |
| Sulfur | 0.5 to 6% |
| Metals | less than 1000 ppm |
- The hydrocarbons in crude oil
Petroleum is a mixture of a very large number of different hydrocarbons. The most common hydrocarbons found in petroleum crude oil are linear or branched alkanes (also called paraffins), cycloalkanes (also called cyclic paraffins or naphthenes), aromatic hydrocarbons, or much more complicated chemicals like asphaltenes which may have a molecular weight of 800 to 500.[4][5] .
The alkanes present in crude oil are saturated hydrocarbons, with linear or branched chains, which contain only carbon and hydrogen atoms and have the general formula of CnH2n+2. They generally have from 4 to 40 carbon atoms per molecule, although some molecules may be present that have less than 5 or more than 40 carbon atoms.
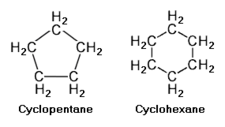
The cycloalkanes are also saturated hydrocarbons, but they which have one or more rings of carbon atoms to which hydrogen atoms are attached. The general formula for cycloalkane having a single ring of carbon atoms (with no side chains) is CnH2n. Cycloalkanes have similar properties to alkanes but have higher boiling points.
The upper adjacent diagram depicts the chemical structure of cyclopentane and cyclohexane as examples of cycloalkanes having a single ring.
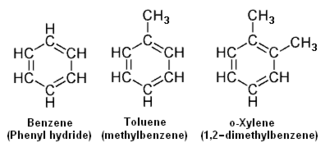
The aromatic hydrocarbons have one or more rings of six carbons, called benzene rings, to which hydrogen atoms are attached. The general formula of the aromatic hydrocarbons having a single ring (with no side chains) is
CnHn.
The lower adjacent diagram depicts the chemical structures of benzene as an example of an aromatic hydrocarbon having a single ring with no side chains, as well as the structures of toluene and o-Xylene as examples of aromatic hydrocarbons having a single benzene ring with one and with two side chains.
Products produced from crude petroleum
The petroleum crude oil is refined in petroleum refineries to produce various fuels as well as a number of other products.
- Fuels
- Liquified petroleum gas, commonly referred to as LPG
- Gasoline, also called petrol, in various grades
- Jet fuel in various grades
- Kerosene
- Diesel fuel
- Fuel oil
- Other products
- Solvents for various industrial and other uses
- Lubricants such as motor oils and greases
- Petroleum wax
- Sulfur, a byproduct of sulfur removal from fuels.
- Asphalt
- Petroleum coke, used in speciality carbon products or as a solid fuel.
- Petrochemical feed stocks:
- Benzene, toluene and xylenes
- Petroleum naphtha and fuel oils as feedstocks for steam-assisted thermal cracking plants referred to as steam crackers that produce intermediate petrochemical feedstocks
Formation
According to generally accepted theory, petroleum is derived from ancient biomass.[6] The theory was initially based on the isolation of molecules from petroleum that closely resemble known biomolecules (Figure).
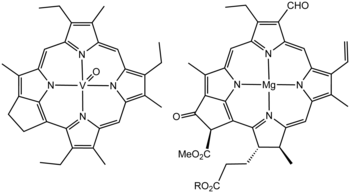
More specifically, crude oil and natural gas are products of heating of ancient organic materials (i.e. kerogen) over geological time. Formation of petroleum occurs from hydrocarbon pyrolysis, in a variety of mostly endothermic reactions at high temperature and/or pressure.[7] Today's oil formed from the preserved remains of prehistoric zooplankton and algae, which had settled to a sea or lake bottom in large quantities under anoxic conditions (the remains of prehistoric terrestrial plants, on the other hand, tended to form coal). Over geological time the organic matter mixed with mud, and was buried under heavy layers of sediment resulting in high levels of heat and pressure (known as diagenesis). This process caused the organic matter to change, first into a waxy material known as kerogen, which is found in various oil shales around the world, and then with more heat into liquid and gaseous hydrocarbons via a process known as catagenesis.
Geologists often refer to the temperature range in which oil forms as an "oil window"—below the minimum temperature oil remains trapped in the form of kerogen, and above the maximum temperature the oil is converted to natural gas through the process of thermal cracking. Although this temperature range is found at different depths below the surface throughout the world, a typical depth for the oil window is 4–6 km. Sometimes, oil which is formed at extreme depths may migrate and become trapped at much shallower depths than where it was formed. The Athabasca Oil Sands is one example of this.
Crude oil
Conventional crude oil reservoirs
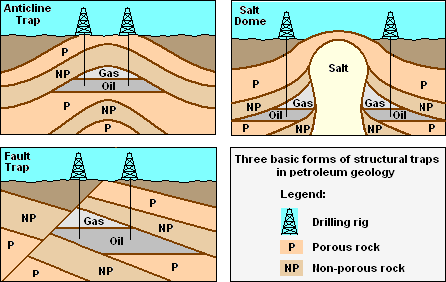
Three conditions must be present for oil reservoirs to form, as depicted in the adjacent drawing:
- A reservoir of hydrocarbon material must exist and must have been buried deep enough for subterranean heat and pressure to have transformed it over a long period of time into crude oil.
- A porous, permeable reservoir rock for the crude oil to accumulate in.
- A non-porous, non-permeable cap rock that acts to seal and to prevent the accumulated crude oil from migrating upward and escaping to the surface.
Because most hydrocarbons are lighter than rock or water, they often migrate upward by permeating through porous, permeable rock layers until either reaching the surface or becoming trapped by non-porous, impermeable rocks above. When hydrocarbons are accumulated in a such a trap, an oil reservoir forms from which the oil can be extracted by drilling and pumping as also shown in the adjacent drawing.
Unconventional oil reservoirs
Oil-eating bacteria biodegrades oil that has escaped to the surface. Oil sands are reservoirs of partially biodegraded oil still in the process of escaping and being biodegraded, but they contain so much migrating oil that, although most of it has escaped, vast amounts are still present—more than can be found in conventional oil reservoirs. The lighter fractions of the crude oil are destroyed first, resulting in reservoirs containing an extremely heavy form of crude oil, called crude bitumen in Canada, or extra-heavy crude oil in Venezuela. These two countries have the world's largest deposits of oil sands.
On the other hand, oil shales are source rocks that have not been exposed to heat or pressure long enough to convert their trapped hydrocarbons into crude oil. Technically speaking, oil shales are not really shales and do not really contain oil, but are usually relatively hard rocks called marls containing a waxy substance called kerogen. The kerogen trapped in the rock can be converted into crude oil using heat and pressure to simulate natural processes. The method has been known for centuries and was patented in 1694 under British Crown Patent No. 330 covering, "A way to extract and make great quantityes of pitch, tarr, and oyle out of a sort of stone." Although oil shales are found in many countries, the United States has the world's largest deposits.[8]
Classification
The petroleum industry generally classifies crude oil by the geographic location of the reservoir from which it is produced (e.g. West Texas Intermediate, Brent, or Oman), its API gravity (an oil industry measure of density), and by its sulfur content. Crude oil may be considered light if it has a low density or heavy if it has a high density and it may be referred to as sweet if it contains relatively little sulfur or sour if it contains substantial amounts of sulfur.
Light crude oil is more desirable than heavy oil since it provides a higher yield of gasoline and sweet oil is more desirable than sour oil because it has fewer environmental problems and requires less refining to meet sulfur content standards of refined fuels. Each crude oil has a unique composition and set of physical properties which are delineated by crude oil assays performed in petroleum laboratories.
Some of the common petroleum crude oils are:
- West Texas Intermediate is a very high-quality, sweet, light oil. It is a North American oil.
- Brent Blend is a blend of 15 oils from the Brent and Ninian oil fields in the East Shetland Basin of the North Sea.
- Dubai Crude is a Middle Eastern, sour oil from Dubai.
- Arabian Crude is a Middle Eastern oil from Saudi Arabia.
- Tapis Crude]] is a Far Eastern oil from Malaysia.
- Minas Crude is a Far Eastern oil from Indonesia.
- Canada (to be added)
- Russia (to be added)
Petroleum industry
The petroleum industry is involved in the global processes of exploration, extraction, refining, transporting (often with oil tankers and pipelines), and marketing petroleum products. The largest volume products of the industry are fuel oil and gasoline (petrol). Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics. The industry is usually divided into three major components: upstream, midstream and downstream. Midstream operations are usually included in the downstream category.
Petroleum is vital to many industries, and is of importance to the maintenance of industrialized civilization itself, and thus is critical concern to many nations. Oil accounts for a large percentage of the world's energy consumption, ranging from a low of 32% for Europe and Asia, up to a high of 53% for the Middle East. Other geographic regions' consumption patterns are as follows: South and Central America (44%), Africa (41%), and North America (40%). The world at large consumes 30 billion barrels (4.8 km³) of oil per year, and the top oil consumers largely consist of developed nations. In fact, 24% of the oil consumed in 2004 went to the United States alone [9], though by 2007 this had dropped to 21% of world oil consumed.[10]
In the US, in the states of Arizona, California, Hawaii, Nevada, Oregon and Washington, the Western States Petroleum Association (WSPA) is responsible for producing, distributing, refining, transporting and marketing petroleum. This non-profit trade association was founded in 1907, and is the oldest petroleum trade association in the United States.[11]
History
Petroleum, in one form or another, has been used since ancient times, and is now important across society, including in economy, politics and technology. The rise in importance was mostly due to the invention of the internal combustion engine.
More than 4000 years ago, according to Herodotus and Diodorus Siculus, asphalt was used in the construction of the walls and towers of Babylon; there were oil pits near Ardericca (near Babylon), and a pitch spring on Zacynthus.[12] Great quantities of it were found on the banks of the river Issus, one of the tributaries of the Euphrates. Ancient Persian tablets indicate the medicinal and lighting uses of petroleum in the upper levels of their society.
Today, about 90% of vehicular fuel needs are met by oil. Petroleum also makes up 40% of total energy consumption in the United States, but is responsible for only 2% of electricity generation. Petroleum's worth as a portable, dense energy source powering the vast majority of vehicles and as the base of many industrial chemicals makes it one of the world's most important commodities.
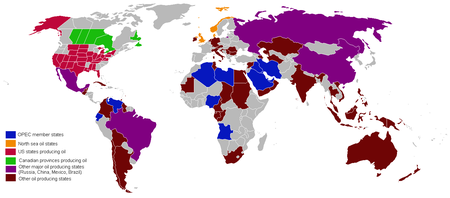
The top three oil producing countries are Saudi Arabia, Russia, and the United States.[13] About 80% of the world's readily accessible reserves are located in the Middle East, with 62.5% coming from the Arab 5: Saudi Arabia, UAE, Iraq, Qatar and Kuwait. A large portion of the world's total oil exists as unconventional sources, such as bitumen in Canada and Venezuela and oil shale. While significant volumes of oil are extracted from oil sands, particularly in Canada, logistical and technical hurdles remain, and Canada's oil sands are not expected to provide more than a few million barrels per day in the foreseeable future.
Petroleum by country
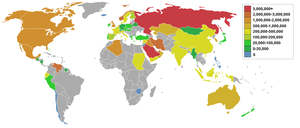
Consumption statistics
Consumption
This table orders the amount of petroleum consumed in 2006 in thousand barrels (bbl) per day and in thousand cubic metres (m3) per day:[14][15][16]
| Consuming Nation 2006 | (1000 bbl/day) | (1000 m3/day) | population in millions | bbl/year per capita |
|---|---|---|---|---|
| Template:Rh|United States 1 | 20687.42 oilbbl (Expression error: Missing operand for round. m3) | 304 | Template:Round | |
| Template:Rh|China | 7201.28 oilbbl (Expression error: Missing operand for round. m3) | 1369 | Template:Round | |
| Template:Rh|Japan 2 | 5197.70 oilbbl (Expression error: Missing operand for round. m3) | 128 | Template:Round | |
| Template:Rh|Russia 1 | 2810.76 oilbbl (Expression error: Missing operand for round. m3) | 142 | Template:Round | |
| Template:Rh|Germany 2 | 2691.81 oilbbl (Expression error: Missing operand for round. m3) | 82 | Template:Round | |
| Template:Rh|India 2 | 2571.90 oilbbl (Expression error: Missing operand for round. m3) | 1201 | Template:Round | |
| Template:Rh|Canada | 2296.66 oilbbl (Expression error: Unrecognized word "m". 1) | 32[17] | Template:Round | |
| Template:Rh|Brazil | 2216.84 oilbbl (Expression error: Unrecognized word "m". 1) | 187 | Template:Round | |
| Template:Rh|South Korea 2 | 2179.90 oilbbl (Expression error: Unrecognized word "m". 1) | 49[18] | Template:Round | |
| Template:Rh|Saudi Arabia (OPEC) | 2139.42 oilbbl (Expression error: Unrecognized word "m". 1) | 27[19] | Template:Round | |
| Template:Rh|Mexico 1 | 2077.51 oilbbl (Expression error: Unrecognized word "m". 1) | 107 | Template:Round | |
| Template:Rh|France 2 | 1981.18 oilbbl (Expression error: Unrecognized word "m". 1) | 61[20] | Template:Round | |
| Template:Rh|United Kingdom 1 | 1812.01 oilbbl (Expression error: Unrecognized word "m". 1) | 61[21] | Template:Round | |
| Template:Rh|Italy 2 | 1742.58 oilbbl (Expression error: Unrecognized word "m". 1) | 58[22] | Template:Round | |
| Template:Rh|Iran (OPEC) | 1679.20 oilbbl (Expression error: Unrecognized word "m". 1) | 68[23] | Template:Round |
Source: US Energy Information Administration
1 peak production of oil already passed in this state
2 This country is not a major oil producer
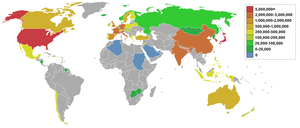
Production
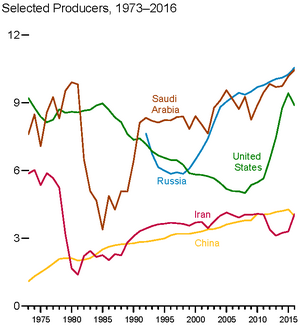
In petroleum industry parlance, production refers to the quantity of crude extracted from reserves, not the literal creation of the product.
Source: U.S. Energy Information Administration
1 Peak production of conventional oil already passed in this state.
2 Although Canadian conventional oil production is declining, total oil production is increasing as oil sands production grows. If oil sands are included, it has the world's second largest oil reserves after Saudi Arabia.
3 Though still a member, Iraq has not been included in production figures since 1998
Export
See also: Fossil fuel exporters
In order of net exports in 2006 in thousand bbl/d and thousand m³/d:
| # | Exporting Nation (2006) | (103bbl/d) | (103m3/d) |
|---|---|---|---|
| 1 | Template:Rh|Saudi Arabia (OPEC) | 8,651 | 1,376 |
| 2 | Template:Rh|Russia 1 | 6,565 | 1,044 |
| 3 | Template:Rh|Norway 1 | 2,542 | 404 |
| 4 | Template:Rh|Iran (OPEC) | 2,519 | 401 |
| 5 | Template:Rh|United Arab Emirates (OPEC) | 2,515 | 400 |
| 6 | Template:Rh|Venezuela (OPEC) 1 | 2,203 | 350 |
| 7 | Template:Rh|Kuwait (OPEC) | 2,150 | 342 |
| 8 | Template:Rh|Nigeria (OPEC) | 2,146 | 341 |
| 9 | Template:Rh|Algeria (OPEC) 1 | 1,847 | 297 |
| 10 | Template:Rh|Mexico 1 | 1,676 | 266 |
| 11 | Template:Rh|Libya (OPEC) 1 | 1,525 | 242 |
| 12 | Template:Rh|Iraq (OPEC) | 1,438 | 229 |
| 13 | Template:Rh|Angola (OPEC) | 1,363 | 217 |
| 14 | Template:Rh|Kazakhstan | 1,114 | 177 |
| 15 | Template:Rh|Canada 2 | 1,071 | 170 |
Source: US Energy Information Administration
1 peak production already passed in this state
2 Canadian statistics are complicated by the fact it is both an importer and exporter of crude oil, and refines large amounts of oil for the U.S. market. It is the leading source of U.S. imports of oil and products, averaging 2.5 MMbbl/d in August 2007. [1].
Total world production/consumption (as of 2005) is approximately 84 Moilbbl/d (Expression error: Missing operand for round. m3/d).
See also: Organization of Petroleum Exporting Countries.
Import
In order of net imports in 2006 in thousand bbl/d and thousand m³/d:
| # | Importing Nation (2006) | (103bbl/day) | (103m3/day) |
|---|---|---|---|
| 1 | Template:Rh|United States 1 | 12,220 | 1,943 |
| 2 | Template:Rh|Japan | 5,097 | 810 |
| 3 | Template:Rh|China 2 | 3,438 | 547 |
| 4 | Template:Rh|Germany | 2,483 | 395 |
| 5 | Template:Rh|South Korea | 2,150 | 342 |
| 6 | Template:Rh|France | 1,893 | 301 |
| 7 | Template:Rh|India | 1,687 | 268 |
| 8 | Template:Rh|Italy | 1,558 | 248 |
| 9 | Template:Rh|Spain | 1,555 | 247 |
| 10 | Template:Rh|Republic of China (Taiwan) | 942 | 150 |
| 11 | Template:Rh|Netherlands | 936 | 149 |
| 12 | Template:Rh|Singapore | 787 | 125 |
| 13 | Template:Rh|Thailand | 606 | 96 |
| 14 | Template:Rh|Turkey | 576 | 92 |
| 15 | Template:Rh|Belgium | 546 | 87 |
Source: US Energy Information Administration
1 peak production of oil already passed in this state
2 Major oil producer whose production is still increasing
Non-producing consumers
Countries whose oil production is 10% or less of their consumption.
| # | Consuming Nation | (bbl/day) | (m³/day) |
|---|---|---|---|
| 1 | Template:Rh|Japan | 5,578,000 | 886,831 |
| 2 | Template:Rh|Germany | 2,677,000 | 425,609 |
| 3 | Template:Rh|South Korea | 2,061,000 | 327,673 |
| 4 | Template:Rh|France | 2,060,000 | 327,514 |
| 5 | Template:Rh|Italy | 1,874,000 | 297,942 |
| 6 | Template:Rh|Spain | 1,537,000 | 244,363 |
| 7 | Template:Rh|Netherlands | 946,700 | 150,513 |
Source : CIA World Factbook
Environmental effects
The presence of oil has significant social and environmental impacts, from accidents and routine activities such as seismic exploration, drilling, and generation of polluting wastes, greenhouse gases and climate change not produced by renewable energy.
Extraction
Oil extraction is costly and sometimes environmentally damaging, although Dr. John Hunt of the Woods Hole Oceanographic Institution pointed out in a 1981 paper that over 70% of the reserves in the world are associated with visible macroseepages, and many oil fields are found due to natural seeps. Offshore exploration and extraction of oil disturbs the surrounding marine environment.[25] Extraction may involve dredging, which stirs up the seabed, killing the sea plants that marine creatures need to survive. But at the same time, offshore oil platforms also form micro-habitats for marine creatures.
Oil spills
Crude oil and refined fuel spills from tanker ship accidents have damaged natural ecosystems in Alaska, the Galapagos Islands, France and many other places. The quantity of oil spilled during accidents has ranged from a few hundred tons to several hundred thousand tons (e.g., Atlantic Empress, Amoco Cadiz). Smaller spills have already proven to have a great impact on ecosystems, such as the Exxon Valdez oil spill
Oil spills at sea are generally much more damaging than those on land, since they can spread for hundreds of nautical miles in a thin oil slick which can cover beaches with a thin coating of oil. This can kill sea birds, mammals, shellfish and other organisms it coats. Oil spills on land are more readily containable if a makeshift earth dam can be rapidly bulldozed around the spill site before most of the oil escapes, and land animals can avoid the oil more easily.
Control of oil spills is difficult, requires ad hoc methods, and often a large amount of manpower (picture). The dropping of bombs and incendiary devices from aircraft on the Torrey Canyon wreck produced poor results;[26] modern techniques would include pumping the oil from the wreck, like in the Prestige oil spill or the Erika oil spill.[27]
Future of petroleum production
USA Today news reported in 2004 that there were 40 years of petroleum left in the ground. As similar statements have been made in the 40 previous years, it hardly carries the complex situation.
Consumption in the twentieth century has been abundantly pushed by automobile growth ; the 1985-2003 oil glut even fuelled the sales of low economy vehicles (SUVs) in OECD countries. In 2008, the economic crisis seems to have some impact on the sales of such vehicles ; still, the 2008 oil consumption shows a small increase. The BRIC countries might also kick in, as China briefly was the first automobile market in december 2009[28] . The immediate outlook still hints upwards. In the long term, uncertainties loiter ; the OPEC believes that the OECD countries will push low consumption policies at some point in the future ; when that happens, it will definitely curb the oil sales, and both OPEC and EIA kept lowering their 2020 consumption estimates during the past 5 years [29]. Oil products are more and more in competition with alternative sources, mainly coal and natural gas, both cheaper sources.
Production will also face an increasingly complex situation ; while OPEC countries still have large reserves at low production prices, newly found reservoirs often lead to higher prices ; offshore giants such as Tupi, Guara and Tiber demand high investments and ever-increasing technological abilities. Subsalt reservoirs such as Tupi were unknown in the twentieth century, mainly because the industry was unable to probe them. Enhanced Oil Recovery (EOR) techniques (example : DaQing, China [30] ) will continue to play a major role in increasing the world's recoverable oil.
Hubbert peak theory
The Hubbert peak theory (also known as peak oil) posits that future petroleum production (whether for individual oil wells, entire oil fields, whole countries, or worldwide production) will eventually peak and then decline at a similar rate to the rate of increase before the peak as these reserves are exhausted. The peak of oil discoveries was in 1965, and oil production per year has surpassed oil discoveries every year since 1980.[31]
Controversy surrounds predictions of the timing of the global peak, as these predictions are dependent on the past production and discovery data used in the calculation as well as how unconventional reserves are considered. Also, these predictions do not take into account outside elements such as the current economic crisis (2008). Also, many Peak Oil promoters proposed many different dates, some of them passed already .
It is difficult to predict the oil peak in any given region, due to the lack of knowledge and/or transparency in accounting of global oil reserves.[32] Based on available production data, proponents have previously predicted the peak for the world to be in years 1989, 1995, or 1995-2000. Some of these predictions date from before the recession of the early 1980s, and the consequent reduction in global consumption, the effect of which was to delay the date of any peak by several years. Just as the 1971 U.S. peak in oil production was only clearly recognized after the fact, a peak in world production will be difficult to discern until production clearly drops off.
References
- ↑ Bauer Georg (1546). De Natura Fossilium. Translated in 1955 by Mark C. Bandy and Jean A. Bandy
- ↑ Norman J. Hyne (2001). Nontechnical Guide to Petroleum Geology, Exploration, Drilling, and Production. PennWell Corporation, pages 1-4. ISBN 087814823X.
- ↑ Speight, James G. (1999). The Chemistry and Technology of Petroleum. Marcel Dekker, 215–216. ISBN 0824702174.
- ↑ Oliver Mullins and Eric Sheu (Editors) (1999). Structure & Dynamics of Asphaltenes, 1st Edition. Springer. ISBN 0-306-45930-2. (See Chapter 1, page 17)
- ↑ Note: There are many other values in the technical literature for the molecular weight of asphaltenes and there does not appear to be a concensus as to which values are more correct.
- ↑ Keith A. Kvenvolden “Organic geochemistry – A retrospective of its first 70 years” Organic Geochemistry 37 (2006) 1–11. Template:DOI
- ↑ Petroleum Study
- ↑ {{cite news | title=Oil Shale: Ready to Unlock the Rock | first=Giles | last=Lambertson | publisher=Construction Equipment Guide | url=http://www.cegltd.com/story.asp?story=10092 | date=February 16, 2008
- ↑ International Energy Annual 2004 (XLS). Energy Information Administration (2006-07-14).
- ↑ Yearbook 2008 - crude oil. Enerdata.
- ↑ Western States Petroleum Association - About Us. Retrieved on 2008-11-03.
- ↑ This article incorporates text from the Encyclopædia Britannica Eleventh Edition article "Petroleum", a publication now in the public domain.
- ↑ InfoPlease
- ↑ U.S. Energy Information Administration. Excel file RecentPetroleumConsumptionBarrelsperDay.xls from web page http://tonto.eia.doe.gov/dnav/pet/pet_pri_wco_k_w.htm (direct link: http://www.eia.doe.gov/emeu/international/RecentPetroleumConsumptionBarrelsperDay.xls) "Table Posted: November 7, 2008"
- ↑ From DSW-Datareport 2006 ("Deutsche Stiftung Weltbevölkerung")
- ↑ One cubic metre of oil is equivalent to 6.28981077 barrels of oil
- ↑ Beauchesne, Eric (2007-03-13). We are 31,612,897. National Post. Retrieved on 2008-11-11.
- ↑ IndexMundi. South Korea Population - Demographics. "48,846,823" ... "July 2006 est." Retrieved 2008-11-11
- ↑ Sources vary: 24,600,000 from UNHCR / Refworld / The Worst of the Worst 2006 - Saudi Arabia. United Nations High Commissioner for Refugees. Retrieved on 2008-11-11.; while IndexMundi listed a July 2006 estimate of 27,019,73: Saudi Arabia Population - Demographics. IndexMundi. Retrieved on 2008-11-11.
- ↑ IndexMundi. France Population - Demographics. "60,876,136" ... "July 2006 est." Retrieved 2008-11-11
- ↑ IndexMundi. United Kingdom Population - Demographics. "60,609,153" ... "July 2006 est." Retrieved 2008-11-11
- ↑ IndexMundi. Italy Population - Demographics. "58,133,509" ... "July 2006 est." Retrieved 2008-11-11
- ↑ IndexMundi. Iran Population - Demographics. "68,688,433" ... "July 2006 est." Retrieved 2008-11-11
- ↑ http://www.eia.doe.gov/emeu/aer/pdf/pages/sec11_10.pdf
- ↑ Waste discharges during the offshore oil and gas activity by Stanislave Patin, tr. Elena Cascio
- ↑ Torrey Canyon bombing by the Navy and RAF
- ↑ Pumping of the Erika cargo
- ↑ Chris Hogg (2009-02-10). China's car industry overtakes US.
- ↑ OPEC Secretariat (2008). World Oil Outlook 2008.
- ↑ Ni Weiling (2006-10-16). Daqing Oilfield rejuvenated by virtue of technology.
- ↑ Campbell CJ (2000-12). Peak Oil Presentation at the Technical University of Clausthal.
- ↑ New study raises doubts about Saudi oil reserves