Trypanosoma brucei: Difference between revisions
imported>Shirley Chan No edit summary |
imported>Meg Taylor m (spelling: dicovered -> discovered) |
||
| (81 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{ | {{TOC|left}} | ||
{{Taxobox | {{Taxobox | ||
| color = pink | | color = pink | ||
| Line 17: | Line 16: | ||
}} | }} | ||
'''''Trypanosoma brucei''''' is a unicellular [[parasitic]] organism with no true tissues. It belongs to the [[protista]] [[kingdom]] and therefore has cell structures that are similar to the cells of many [[eukaryotes]]. [[T. brucei]] uses [[tsetse flies]] as vectors to carry the parasite and transmit diseases through the flies' blood meals. Tsetse flies are often found in moist [[woodlands]], [[savannas]], and underdeveloped rural areas of sub-saharan Africa. In 1803, an English doctor, Thomas M. Winterbottom, made observations of sick people who had frequent symptom of swollen cervical lymph nodes. However, the cause of the disease still remained unknown. In 1843, a parasite was discovered in the blood of a frog and it was the first time the parasite was named "[[trypanosome]]". Later in 1881, the same parasite was found in the blood of sick camels and horses. In 1895, Dr. David Bruce, a British army medical officer, discovered a species of trypanosome that caused the cattle disease called [[nagana]] in South Africa. Dr. Bruce named the species after him and therefore the name "''trypanosoma brucei''". It was not till 1902 that the first motile parasite of [[trypanosome]] was identified in human. The parasite ''T.brucei'' was found to be the cause of a tropical disease named "[[trypanosomiasis]]", which is also called the [[African Sleeping Sickness]]. Sleeping sickness spreads easily to human and animals in rural areas that have weak health systems. Failure in controlling the spread of the disease and enhancing local healthcare needs have caused an increased impact of the parasite in both humans and livestocks in underdeveloped regions of Africa. According to the [[World Health Organization]], there are approximately 50,000-70,000 cases of [[trypanosomiasis]] found in Western and Central Africa<ref>World Health Organization. Avenue Appia 20�CH - 1211 Geneva 27, Switzerland. +41 22 791 2111. <http://www.who.int/tdr/diseases/tryp/default.htm></ref>. No one is immuned from this disease and it can be possible for one to get re-infected. Infection risk increases with the number of times a person gets bitten by the infected tsetse flies. Understanding the species of ''T. brucei'' will allow us to improve public health and cure for the sickness this organism causes in underdeveloped country of Africa.<br /> | |||
''Trypanosoma brucei'' is a unicellular [[parasitic]] organism with no true tissues. It belongs to the [[protista]] [[kingdom]] and therefore has cell structures that are similar to the cells of many [[eukaryotes]]. | |||
==Genome structure== | ==Genome structure== | ||
The Trypanosoma brucei genome was sequenced by the Institute for Genomic Research and the Sanger Institute. This organism is made up of a two unit genome: a nuclear genome and a mitochondrial genome. The nuclear genome houses linear DNA molecules and are composed of 85% of the total cellular DNA. The nuclear genome has chromosomes that are divided into three types based on their sizes. There are 11 large chromosomes of 1-6Mb, 6 intermediate chromosomes of 300-600Kb, and 100 minichromosomes of 50-100kb. The intermediate chromosomes are found to play a role in antigenic variation, which is useful for T.brucei to escape the immune system of the host. | The ''Trypanosoma brucei'' genome was sequenced by the [http://www.tigr.org/tdb/e2k1/tba1/ Institute for Genomic Research]and the [http://www.sanger.ac.uk/Projects/T_brucei/ Sanger Institute]. This organism is made up of a two unit [[genome]]: a nuclear genome and a mitochondrial genome. The nuclear genome houses linear DNA molecules and are composed of 85% of the total cellular DNA. The nuclear genome has [[chromosomes]] that are divided into three types based on their sizes. There are 11 large chromosomes of 1-6Mb, 6 intermediate chromosomes of 300-600Kb, and 100 minichromosomes of 50-100kb.<ref>Berriman, Matt. The ''Trypanosoma brucei'' Genome Project. [http://www.sanger.ac.uk/Projects/T_brucei/ The Wellcome Trust Sanger Institute]. </ref> The intermediate chromosomes are found to play a role in antigenic variation, which is useful for ''T.brucei'' to escape the immune system of the host. The mitochondrial genome consists of the remaining 15% of the total cellular DNA. Inside the mitochondrial genome, the organism's [[kinetoplast]] is responsible for carrying 25-35 large circular DNA molecules called [[maxicircles]] and thousands of small circular DNA molecules called [[minicircles]]<ref>Black, Samuel J. (2001). World Class Parasites: Volume 1, The African Trypanosomes, First Edition. Kluwer Academic Publishers.</ref>. Both the maxicircles and minicircles are organized into a network of a disk-shape structure, [[nucleoprotein disk]], which is located at the base of the [[flagellum]]. | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
''Trypanosoma brucei'' has organelles including [[endoplasmic reticulum]], [[golgi apparatus]], [[lysosome]], [[nucleus]], and a large mitochondrion. Its [[mitochondrion]] houses a unique structure called [[kinetoplas]]t, where DNA is located. ''T. brucei'' has no definite shape, it is mobile and has a single [[flagellum]] for locomotion. The flagellum runs along the undulating membrane. As the flagellum oscillates, it produces a wavelike motion and pushes the cell along. The base of the flagellum is associated with the kinetoplast in a single large mitochondrion. The [[basal body]] plays a role in organizing spindles during mitosis. ''T. brucei'' is asexual and reproduces by [[binary fission]] through mitotic division. The basal body is the first to replicate, followed by the replication of the kinetoplast within the mitochondrion. As the daughter flagellum grows, the nucleus undergoes [[mitosis]]. After the mitochondrion divides, cytoplasm undergoes [[cytokinesis]] to form two identical cells. <br /><br /> | |||
Because of its animal-like cells, T. brucei has heterotrophic cells that require organic molecules for its source of energy. Studies have shown that it possesses some forms of energy producing enzymes. The organism acquires proline as a major energy source when it lives inside the gut of the tsetse fly. Since there is not much nutrients to be obtained from the gut of an insect, the parasite’s mitochondrion needs to carry out some type of metabolic pathways in order to generate sufficient energy. On the contrary, when nutrients become more abundant in mammalian host, the parasite can rely on the glucose from its host as its source of energy without having to undergo metabolic pathways to produce energy for its own use. This causes a severe malnutrition and rapid weight loss in the host.<br /> | Because of its animal-like cells, ''T. brucei'' has [[heterotrophic]] cells that require organic molecules for its source of energy. Studies<ref>Bacteriol, J.(1992 February) “Mutual adjustment of glucose uptake and metabolism in ''Trypanosoma brucei'' grown in a chemostat”. Research Unit for Tropical Diseases, International Institute for Cellular and Molecular Pathology, Brussels, Belgium, 174(4): 1273–1279. Retrieved March 3, 2008, from PubMed Central Database.</ref> have shown that it possesses some forms of energy producing enzymes. The organism acquires [[proline]] as a major energy source when it lives inside the gut of the tsetse fly. Since there is not much nutrients to be obtained from the gut of an insect, the parasite’s mitochondrion needs to carry out some type of metabolic pathways in order to generate sufficient energy. On the contrary, when nutrients become more abundant in mammalian host, the parasite can rely on the glucose from its host as its source of energy without having to undergo metabolic pathways to produce energy for its own use. This causes a severe [[malnutrition]] and rapid weight loss in the host.<br /> | ||
==Ecology== | ==Ecology== | ||
Life Cycle of Trypanosoma brucei<br /> | Life Cycle of ''Trypanosoma brucei''<ref>"Life Cycle of ''Trypanosoma brucei''." Chart. Retrieved March 3, 2008, from [http://www.dpd.cdc.gov/dpdx/HTML/TrypanosomiasisAfrican.htm Centers for Disease Control and Prevention.] </ref><br /> | ||
[[Image:TbruceiLifeCycle.jpg]]<br /><br /> | [[Image:TbruceiLifeCycle.jpg]]<br /><br /> | ||
During the development of T. brucei, morphological changes play an important role in the organism’s life cycle between procyclic stage and bloodstream stage. The parasite takes the form of procyclic trypomastigote while in the | During the development of ''T. brucei'', morphological changes play an important role in the organism’s life cycle between[[ procyclic]] stage and bloodstream stage. The parasite takes the form of [[procyclic]] [[trypomastigote]] while in the midgut of the tsetse fly. Reproduction and profiliation of ''T. brucei'' take place through [[binary fission]] inside the fly's midgut. Once the parasite migrates to the anterior of the midgut, it transforms into [[epimastigote]]. As epimastigote reaches the fly’s salivary gland, it attaches the flagella to the wall of the salivary gland and undergoes morphological change to form metacyclic trypomastigote, which is a form capable of infecting mammalian hosts. After[[ metacyclic]] [[trypomastigote]] is injected into susceptible host through the tsetse fly’s blood meal, the parasite circulates throughout the body of the host and changes into the form of bloodstream trypomastigotes, a form capable of infecting tsetse flies again.<ref>Centers for Disease Control and Prevention. 1600 Clifton Rd., NE, Atlanta, GA 30333. (800) 311-3435, (404) 639-3311. <http://www.cdc.gov>. | ||
</ref> When a tsetse fly takes a blood meal on the infected host, it becomes infected and goes on to disperse the parasite into the next mammalian host. This becomes a problem when poor communities cannot afford diagnostic tests. Infected individuals might not realize that they have the disease. As a result, each untreated infected human or animal creates a new host for uninfected tsetse fly. Migration of infected individuals from rural areas of Africa also spreads the parasite to other regions. | |||
==Pathology== | ==Pathology== | ||
After the parasite is transmitted into the bloodstream of mammalian host, it can be carried to the lymph and spinal fluid through circulation. ''T. brucei'' begins to replicate in the bloodstream. As the organism migrates to other sites of the body through blood fluids, it begins to invade other body tissues and the [[central nervous system]] of the host. First signs of tsetse fly bites often cause redness, pain and swelling at site of the bite. Other symptoms are headaches, fever, sweating, enlarged lymph nodes and swollen tissues. The infected person will have an uncontrollable urge to sleep due to an increased drowsiness during the day, if symptom gets serious it can lead to coma. As the disease progresses, invasion of CNS can lead to inflmmatioin of the brain and meninges, causing mental deterioration in patients. All of these symptoms can be fatal if not treated.<br /> <br />There are two sub-species of ''T. brucei'': ''T.b.[[rhodesiense]]'' and ''T.b.[[gambiense]]''. ''T.b.rhodesiense'' infects the East and South Africa and represents <10% of the reported cases. This sub-species cause an acute sickness that involves a rapid invasion rate of central nervous system. ''T.b.gambiense'' infects the West and Central Africa and represents more than 90% of the reported cases. This sub-species cause a more chronic infection that usually takes months to years for the infection to reach the central nervous system. Both sub-species can cause diseases that are fatal. <br /> | |||
== | ==Treatments== | ||
There is currently no vaccine available to treat Trypanosomiasis. Diagnostic tests are reliable methods to detect the presence of parasites. Analyzing blood smears, fluids from swollen [[lymph nodes]] and spines are examples of diagnostic methods. Anti-trypanosomal agents such as [[suramin]], [[eflornithine]], [[pentamidine]], and several drugs that contain [[arsenic]] are effective treatments for the disease. However, these drugs are not highly recommendated often because of their significant potential side effects. Patients taking any of these medications should require close monitoring. | |||
==Current Research== | ==Current Research== | ||
1. [http://jcs.biologists.org/cgi/content/full/118/2/283 "The developmental cell biology of ''Trypanosoma brucei''" ]<br /> | |||
<br /> | |||
The organization of cell structures and organelle positioning in ''T. brucei'' are specialized to govern morphological changes needed for the organism to adapt to environmental conditions in different hosts during various stages of the life cycle. The ability to survive in various environments requires a pre-programmed [[differentiation]] of the cell. This research article shows that the trypanosome life cycle interconnects with its whole cell. It puts together basic aspects of ''T. brucei''’s high degree cell structures and their importance in regulating the organism’s cell cycle and developmental functions. Some of the important ideas are that the trypanosome cell is made up of [[microtubule]] [[cytoskeleton]], which underlies the cell membrane and defines the cell shape of the organism. The [[flagellar pocket]], [[flagellum]], [[kinetoplast]], [[mitochondrion]] and [[nucleus]] are located within the cytoskeleton corset. The small size of ''T. brucei'' allows rapid rate of protein trafficking from the inside of the cell to the cell surface. The kinetoplast repositions during ''T.brucei''’s life cycle. Studies have shown that microtubule cytoskeleton extends and allows the posterior end of the cell to grow by 3 µm. The kinetoplast relocates toward the nucleus during DNA replication when the parasite differentiates from the [[stumpy]] form to the procyclic form. The complexity of the genomes and cell division can also be integrated to show how the cell structures are organized to adapt to the various stages of the life cycle.<ref>Matthew, Keith R. “The developmental biology of ''Trypanosoma brucei''.” Institute of Immunology and Infection Research, School of Biological Sciences, University of Edinburgh, West Mains Road, Edinburgh, EH9 3JT, UK. Retrieved March 3, 2008, from Journal of Cell Science 118(2005):238-290. </ref><br /> | |||
2. [http://jcs.biologists.org/cgi/content/full/120/3/478?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=trypanosoma+brucei&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT "Functional genomics in ''Trypanosoma brucei'' identifies evolutionarily conserved components of motile flagella"] | |||
http://jcs.biologists.org/cgi/content/full/ | |||
"Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella" | [[Comparative genomic]] approach is used to identify genes that are associated with the motile flagella in ''Trypanosoma brucei''. It was known that flagella play a multi-functional role in cell locomotion, nutrient uptake, and cell division. However, little was known about the regulation of flagellar beat in organisms. Here, ''T. brucei'' is used as an experimental system to study flagellar biology in eukaryotic cells. The function of flagella has extended to involve cell [[morphogenesis]] and host-parasite interactions in ''T. brucei''. The motile flagella protein TbCMF is being studied in this research in hope to identify components of motile flagella that are evolutionarily conserved. The motile flagella protein TbCMF is composed of 30 novel genes. Mutants with knockout of these genes showed defects in motility. [[Ultrastructural]] analysis was used to identify the TbCMF genes and the results showed that TbCMF genes function to maintain connections between outer doublet microtubules. The results from this experiment give insights to how flagellum is critical for understanding mechanisms in disease pathogenesis and parasite development.<ref>Baron, Desiree M. (2007 January) "Functional genomics in ''Trypanosoma brucei'' identifies evolutionarily conserved components of motile flagella". Molecular Biology Institute, University of California, Los Angeles, CA 90095, USA, Journal of Cell Science 120, 478-491 (2007). Retrieved March 3, 2008, from Journal of Cell Science.</ref> <br /> | ||
3. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1087824 "Mitochondrial DNA Ligases of ''Trypanosoma brucei''" ] | |||
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1087824 | |||
UCLA's research group has done a study on mitochondrial [[DNA ligases]], which play a major role in rejoining the gaps of the newly replicated minicircles in kinetoplast of ''T. brucei''. Both sealing [[Okazaki fragments]] and sealing the minicircles at the terminal step of the DNA replication require the enzyme DNA ligase. The models from the experiment confirmed the presence of two DNA ligase activities associated with the kinetoplast. The kinetoplastid genome was found to have DNA ligase genes LIG kα and LIG kß that not only are transported to the mitochondrion of the organism, but are also localized throughout the mitochondrial genome. The unique form of kinetoplast DNA indicates evolutionary divergence from higher eukaryotes. The gene LIG kß works with other repair enzymes and is most likely involved in joining Okazaki fragments on minicircles. A knockout of LIG kα gene has shown to inhibit ligation and stop the network in releasing covalently closed minicircles. This results in an accumulation of gapped minicircles, a reduction in size of the DNA network, and eventually a loss of DNA within the kinetoplast. The results from this experiment have suggested that LIG kα gene is responsible for the final sealing of gaps in minicircles at the [[origin of replication]] before cleavage of the double-size kinetoplast DNA network. <ref>Downey, Nick. (2005 April) "Mitochondrial DNA Ligases of ''Trypanosoma brucei''". American Society for Microbiology, Molecular Biology Institute and Department of Microbiology, Immunology, and Molecular Genetics, University of California, Los Angeles, California. 4(4): 765–774. Retrieved March 3, 2008, from PubMed Central Database.</ref> | |||
<br /> | |||
==References== | ==References== | ||
<references/> | |||
< | |||
Prescott, Harley, Klein. (2005). Microbiology, Six Edition. New York: McGraw Hill Companies (pp.569-573) | Prescott, Harley, Klein. (2005). Microbiology, Six Edition. New York: McGraw Hill Companies (pp.569-573) | ||
<br /><br /> | |||
Revision as of 23:56, 6 February 2010
| Trypanosoma brucei | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
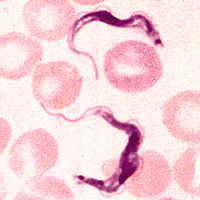 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Trypanosoma brucei |
Trypanosoma brucei is a unicellular parasitic organism with no true tissues. It belongs to the protista kingdom and therefore has cell structures that are similar to the cells of many eukaryotes. T. brucei uses tsetse flies as vectors to carry the parasite and transmit diseases through the flies' blood meals. Tsetse flies are often found in moist woodlands, savannas, and underdeveloped rural areas of sub-saharan Africa. In 1803, an English doctor, Thomas M. Winterbottom, made observations of sick people who had frequent symptom of swollen cervical lymph nodes. However, the cause of the disease still remained unknown. In 1843, a parasite was discovered in the blood of a frog and it was the first time the parasite was named "trypanosome". Later in 1881, the same parasite was found in the blood of sick camels and horses. In 1895, Dr. David Bruce, a British army medical officer, discovered a species of trypanosome that caused the cattle disease called nagana in South Africa. Dr. Bruce named the species after him and therefore the name "trypanosoma brucei". It was not till 1902 that the first motile parasite of trypanosome was identified in human. The parasite T.brucei was found to be the cause of a tropical disease named "trypanosomiasis", which is also called the African Sleeping Sickness. Sleeping sickness spreads easily to human and animals in rural areas that have weak health systems. Failure in controlling the spread of the disease and enhancing local healthcare needs have caused an increased impact of the parasite in both humans and livestocks in underdeveloped regions of Africa. According to the World Health Organization, there are approximately 50,000-70,000 cases of trypanosomiasis found in Western and Central Africa[1]. No one is immuned from this disease and it can be possible for one to get re-infected. Infection risk increases with the number of times a person gets bitten by the infected tsetse flies. Understanding the species of T. brucei will allow us to improve public health and cure for the sickness this organism causes in underdeveloped country of Africa.
Genome structure
The Trypanosoma brucei genome was sequenced by the Institute for Genomic Researchand the Sanger Institute. This organism is made up of a two unit genome: a nuclear genome and a mitochondrial genome. The nuclear genome houses linear DNA molecules and are composed of 85% of the total cellular DNA. The nuclear genome has chromosomes that are divided into three types based on their sizes. There are 11 large chromosomes of 1-6Mb, 6 intermediate chromosomes of 300-600Kb, and 100 minichromosomes of 50-100kb.[2] The intermediate chromosomes are found to play a role in antigenic variation, which is useful for T.brucei to escape the immune system of the host. The mitochondrial genome consists of the remaining 15% of the total cellular DNA. Inside the mitochondrial genome, the organism's kinetoplast is responsible for carrying 25-35 large circular DNA molecules called maxicircles and thousands of small circular DNA molecules called minicircles[3]. Both the maxicircles and minicircles are organized into a network of a disk-shape structure, nucleoprotein disk, which is located at the base of the flagellum.
Cell structure and metabolism
Trypanosoma brucei has organelles including endoplasmic reticulum, golgi apparatus, lysosome, nucleus, and a large mitochondrion. Its mitochondrion houses a unique structure called kinetoplast, where DNA is located. T. brucei has no definite shape, it is mobile and has a single flagellum for locomotion. The flagellum runs along the undulating membrane. As the flagellum oscillates, it produces a wavelike motion and pushes the cell along. The base of the flagellum is associated with the kinetoplast in a single large mitochondrion. The basal body plays a role in organizing spindles during mitosis. T. brucei is asexual and reproduces by binary fission through mitotic division. The basal body is the first to replicate, followed by the replication of the kinetoplast within the mitochondrion. As the daughter flagellum grows, the nucleus undergoes mitosis. After the mitochondrion divides, cytoplasm undergoes cytokinesis to form two identical cells.
Because of its animal-like cells, T. brucei has heterotrophic cells that require organic molecules for its source of energy. Studies[4] have shown that it possesses some forms of energy producing enzymes. The organism acquires proline as a major energy source when it lives inside the gut of the tsetse fly. Since there is not much nutrients to be obtained from the gut of an insect, the parasite’s mitochondrion needs to carry out some type of metabolic pathways in order to generate sufficient energy. On the contrary, when nutrients become more abundant in mammalian host, the parasite can rely on the glucose from its host as its source of energy without having to undergo metabolic pathways to produce energy for its own use. This causes a severe malnutrition and rapid weight loss in the host.
Ecology
Life Cycle of Trypanosoma brucei[5]
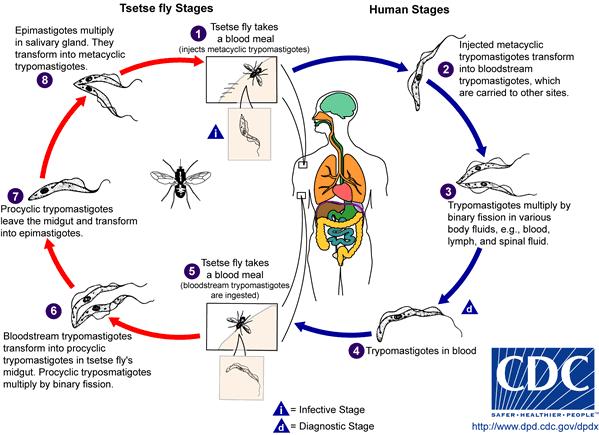
During the development of T. brucei, morphological changes play an important role in the organism’s life cycle betweenprocyclic stage and bloodstream stage. The parasite takes the form of procyclic trypomastigote while in the midgut of the tsetse fly. Reproduction and profiliation of T. brucei take place through binary fission inside the fly's midgut. Once the parasite migrates to the anterior of the midgut, it transforms into epimastigote. As epimastigote reaches the fly’s salivary gland, it attaches the flagella to the wall of the salivary gland and undergoes morphological change to form metacyclic trypomastigote, which is a form capable of infecting mammalian hosts. Aftermetacyclic trypomastigote is injected into susceptible host through the tsetse fly’s blood meal, the parasite circulates throughout the body of the host and changes into the form of bloodstream trypomastigotes, a form capable of infecting tsetse flies again.[6] When a tsetse fly takes a blood meal on the infected host, it becomes infected and goes on to disperse the parasite into the next mammalian host. This becomes a problem when poor communities cannot afford diagnostic tests. Infected individuals might not realize that they have the disease. As a result, each untreated infected human or animal creates a new host for uninfected tsetse fly. Migration of infected individuals from rural areas of Africa also spreads the parasite to other regions.
Pathology
After the parasite is transmitted into the bloodstream of mammalian host, it can be carried to the lymph and spinal fluid through circulation. T. brucei begins to replicate in the bloodstream. As the organism migrates to other sites of the body through blood fluids, it begins to invade other body tissues and the central nervous system of the host. First signs of tsetse fly bites often cause redness, pain and swelling at site of the bite. Other symptoms are headaches, fever, sweating, enlarged lymph nodes and swollen tissues. The infected person will have an uncontrollable urge to sleep due to an increased drowsiness during the day, if symptom gets serious it can lead to coma. As the disease progresses, invasion of CNS can lead to inflmmatioin of the brain and meninges, causing mental deterioration in patients. All of these symptoms can be fatal if not treated.
There are two sub-species of T. brucei: T.b.rhodesiense and T.b.gambiense. T.b.rhodesiense infects the East and South Africa and represents <10% of the reported cases. This sub-species cause an acute sickness that involves a rapid invasion rate of central nervous system. T.b.gambiense infects the West and Central Africa and represents more than 90% of the reported cases. This sub-species cause a more chronic infection that usually takes months to years for the infection to reach the central nervous system. Both sub-species can cause diseases that are fatal.
Treatments
There is currently no vaccine available to treat Trypanosomiasis. Diagnostic tests are reliable methods to detect the presence of parasites. Analyzing blood smears, fluids from swollen lymph nodes and spines are examples of diagnostic methods. Anti-trypanosomal agents such as suramin, eflornithine, pentamidine, and several drugs that contain arsenic are effective treatments for the disease. However, these drugs are not highly recommendated often because of their significant potential side effects. Patients taking any of these medications should require close monitoring.
Current Research
1. "The developmental cell biology of Trypanosoma brucei"
The organization of cell structures and organelle positioning in T. brucei are specialized to govern morphological changes needed for the organism to adapt to environmental conditions in different hosts during various stages of the life cycle. The ability to survive in various environments requires a pre-programmed differentiation of the cell. This research article shows that the trypanosome life cycle interconnects with its whole cell. It puts together basic aspects of T. brucei’s high degree cell structures and their importance in regulating the organism’s cell cycle and developmental functions. Some of the important ideas are that the trypanosome cell is made up of microtubule cytoskeleton, which underlies the cell membrane and defines the cell shape of the organism. The flagellar pocket, flagellum, kinetoplast, mitochondrion and nucleus are located within the cytoskeleton corset. The small size of T. brucei allows rapid rate of protein trafficking from the inside of the cell to the cell surface. The kinetoplast repositions during T.brucei’s life cycle. Studies have shown that microtubule cytoskeleton extends and allows the posterior end of the cell to grow by 3 µm. The kinetoplast relocates toward the nucleus during DNA replication when the parasite differentiates from the stumpy form to the procyclic form. The complexity of the genomes and cell division can also be integrated to show how the cell structures are organized to adapt to the various stages of the life cycle.[7]
Comparative genomic approach is used to identify genes that are associated with the motile flagella in Trypanosoma brucei. It was known that flagella play a multi-functional role in cell locomotion, nutrient uptake, and cell division. However, little was known about the regulation of flagellar beat in organisms. Here, T. brucei is used as an experimental system to study flagellar biology in eukaryotic cells. The function of flagella has extended to involve cell morphogenesis and host-parasite interactions in T. brucei. The motile flagella protein TbCMF is being studied in this research in hope to identify components of motile flagella that are evolutionarily conserved. The motile flagella protein TbCMF is composed of 30 novel genes. Mutants with knockout of these genes showed defects in motility. Ultrastructural analysis was used to identify the TbCMF genes and the results showed that TbCMF genes function to maintain connections between outer doublet microtubules. The results from this experiment give insights to how flagellum is critical for understanding mechanisms in disease pathogenesis and parasite development.[8]
3. "Mitochondrial DNA Ligases of Trypanosoma brucei"
UCLA's research group has done a study on mitochondrial DNA ligases, which play a major role in rejoining the gaps of the newly replicated minicircles in kinetoplast of T. brucei. Both sealing Okazaki fragments and sealing the minicircles at the terminal step of the DNA replication require the enzyme DNA ligase. The models from the experiment confirmed the presence of two DNA ligase activities associated with the kinetoplast. The kinetoplastid genome was found to have DNA ligase genes LIG kα and LIG kß that not only are transported to the mitochondrion of the organism, but are also localized throughout the mitochondrial genome. The unique form of kinetoplast DNA indicates evolutionary divergence from higher eukaryotes. The gene LIG kß works with other repair enzymes and is most likely involved in joining Okazaki fragments on minicircles. A knockout of LIG kα gene has shown to inhibit ligation and stop the network in releasing covalently closed minicircles. This results in an accumulation of gapped minicircles, a reduction in size of the DNA network, and eventually a loss of DNA within the kinetoplast. The results from this experiment have suggested that LIG kα gene is responsible for the final sealing of gaps in minicircles at the origin of replication before cleavage of the double-size kinetoplast DNA network. [9]
References
- ↑ World Health Organization. Avenue Appia 20�CH - 1211 Geneva 27, Switzerland. +41 22 791 2111. <http://www.who.int/tdr/diseases/tryp/default.htm>
- ↑ Berriman, Matt. The Trypanosoma brucei Genome Project. The Wellcome Trust Sanger Institute.
- ↑ Black, Samuel J. (2001). World Class Parasites: Volume 1, The African Trypanosomes, First Edition. Kluwer Academic Publishers.
- ↑ Bacteriol, J.(1992 February) “Mutual adjustment of glucose uptake and metabolism in Trypanosoma brucei grown in a chemostat”. Research Unit for Tropical Diseases, International Institute for Cellular and Molecular Pathology, Brussels, Belgium, 174(4): 1273–1279. Retrieved March 3, 2008, from PubMed Central Database.
- ↑ "Life Cycle of Trypanosoma brucei." Chart. Retrieved March 3, 2008, from Centers for Disease Control and Prevention.
- ↑ Centers for Disease Control and Prevention. 1600 Clifton Rd., NE, Atlanta, GA 30333. (800) 311-3435, (404) 639-3311. <http://www.cdc.gov>.
- ↑ Matthew, Keith R. “The developmental biology of Trypanosoma brucei.” Institute of Immunology and Infection Research, School of Biological Sciences, University of Edinburgh, West Mains Road, Edinburgh, EH9 3JT, UK. Retrieved March 3, 2008, from Journal of Cell Science 118(2005):238-290.
- ↑ Baron, Desiree M. (2007 January) "Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella". Molecular Biology Institute, University of California, Los Angeles, CA 90095, USA, Journal of Cell Science 120, 478-491 (2007). Retrieved March 3, 2008, from Journal of Cell Science.
- ↑ Downey, Nick. (2005 April) "Mitochondrial DNA Ligases of Trypanosoma brucei". American Society for Microbiology, Molecular Biology Institute and Department of Microbiology, Immunology, and Molecular Genetics, University of California, Los Angeles, California. 4(4): 765–774. Retrieved March 3, 2008, from PubMed Central Database.
Prescott, Harley, Klein. (2005). Microbiology, Six Edition. New York: McGraw Hill Companies (pp.569-573)