imported>Paul Wormer |
imported>Paul Wormer |
| (18 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| '''Fritz Haber''' (9 December 1868, [[Breslau]] – 29 January 1934, [[Basel]]) was a German chemist. He was awarded the [[Nobel Prize in Chemistry]] in 1918 for the synthesis of [[ammonia]] from the gaseous [[chemical element|elements]] [[hydrogen]] and [[nitrogen]]. [[Ammonia]] is an important feedstock for artificial fertilizers and explosives. In [[World War I]] Haber supported the use of chemical weapons and actively worked on their development. Of Jewish origin, he was forced to resign his position as director of the [[Kaiser Wilhelm Institut]] in 1933, whereupon he left Germany. He died of a heart attack on a visit to Switzerland.
| | ==Parabolic mirror== |
| == Life == | | {{Image|Refl parab.png|right|350px|Fig. 2. Reflection in a parabolic mirror}} |
| ===Youth and education===
| | Parabolic mirrors concentrate incoming vertical light beams in their focus. We show this. |
| Fritz Haber was born into an assimilated Jewish family. His father, Siegfried Haber, ran a business of dye pigments, paints, and pharmaceuticals. For quite a number of years he also served as alderman of Breslau (then a German city, now the Polish city of [[Wrocław]]). At Fritz's birth, serious medical complications occurred and his mother, Paula—née Haber, a first cousin of Siegfried—died three weeks later. It seemed that Fritz' father blamed the child for the mother's death. This probably was the reason that father and son never became close later in life and that tensions between them arose often.
| |
|
| |
|
| Haber attended the humanistic gymnasium St. Elizabeth in Breslau, where the curriculum contained German language and literature, Latin, Greek, mathematics and some physics, but hardly any chemistry. Fritz had a keen interest in chemistry, already as a school boy he performed chemical experiments. After finishing the gymnasium (September 29, 1886 at the age of seventeen)
| | Consider in figure 2 the arbitrary vertical light beam (blue, parallel to the ''y''-axis) that enters the parabola and hits it at point ''P'' = (''x''<sub>1</sub>, ''y''<sub>1</sub>). The parabola (red) has focus in point ''F''. The incoming beam is reflected at ''P'' obeying the well-known law: incidence angle is angle of reflection. The angles involved are with the line ''APT'' which is tangent to the parabola at point ''P''. It will be shown that the reflected beam passes through ''F''. |
| he went to the Friedrich-Wilhelms-Universität—usually referred to as the [[University of Berlin]]—to study chemistry. (This choice was against his father's wishes, who had preferred a commercial education for his son.) The director of the chemistry department of Berlin University, [[August Wilhelm von Hofmann]] close to seventy at the time, was a poor teacher and had ignored the upkeep of the chemistry lab. As a consequence Fritz Haber found his first semester in Berlin rather disappointing and he decided, as was not unusual for 19th century German students, to switch universities. He chose the [[University of Heidelberg]], where he arrived in the summer semester of 1887 and continued his studies under [[Robert Wilhelm Bunsen]]. He did his second through fourth [[semester]] in Heidelberg. From mid 1889 until mid 1890 Haber spent time in the army.
| |
| {{Image|Fritz Haber age 22.jpeg|right|250px|Fritz Haber at age 22}}
| |
| In the fall of 1890 he went back to Berlin, this time to the ''Technische Hochschule'' of [[Charlottenburg]] (now the [[Technical University Berlin]]). He worked here under [[Carl Liebermann]] who had a cross appointment at Berlin University. Charlottenburg did not have the the right to grant doctorates (it received it later, in 1899). Having done his thesis work at Charlottenburg, Haber received formally his doctorate in organic chemistry at the University of Berlin (May 29, 1891) on basis of a thesis entitled ''Über einige Derivate des Piperonal'' (About some derivatives of [[Piperonal]]).
| |
|
| |
|
| After completion of his university studies, Fritz's father, who had not given up his hope that his son would become a business man, insisted that he do a few apprenticeships in chemical industry. Although Fritz Haber acquired a taste for chemical engineering during these apprenticeships, they also bored him and he convinced his father that he should return to academia to advance his technical knowledge. His father agreed that he spend a semester with [[Georg Lunge]], professor of chemical technology and a distant relative of the Habers, at the [[Eidgenössische Technische Hochschule Zürich|Institute of Technology]] in [[Zurich]]. After that he worked for six months in his father's business, which finally made Haber senior realize that his son's talent was not in commerce.
| | Clearly ∠''BPT'' = ∠''QPA'' (they are vertically opposite angles). Further ∠''APQ'' = ∠''FPA'' because the triangles ''FPA'' and ''QPA'' are congruent and hence ∠''FPA'' = ∠''BPT''. |
|
| |
|
| So finally his father agreed that he take up a scientific career and Fritz went to work with [[Ludwig Knorr]] at the [[University of Jena]], publishing with him one paper.<ref>Ludwig Knorr and Fritz Haber, ''Ueber die Konstitution des Diacetbernsteinsäureesters'' (On the constitution of diaceto amber acid ester), Berichte der deutschen chemischen Gesellschaft
| | We prove the congruence of the triangles: By the definition of the parabola the line segments ''FP'' and ''QP'' are of equal length, because the length of the latter segment is the distance of ''P'' to the directrix and the length of ''FP'' is the distance of ''P'' to the focus. The point ''F'' has the coordinates (0,''f'') and the point ''Q'' has the coordinates (''x''<sub>1</sub>, −''f''). The line segment ''FQ'' has the equation |
| Vol. '''27''', pp. 1151 – 1167</ref> . In Jena in 1893, Haber converted to the [[Protestantism|Protestant]]-Christian faith, against his father's wishes.
| | :<math> |
| ===Karslruhe===
| | \lambda\begin{pmatrix}0\\ f\end{pmatrix} + (1-\lambda)\begin{pmatrix}x_1\\ -f\end{pmatrix}, \quad 0\le\lambda\le 1. |
| After one and a half year in Jena, still uncertain whether to devote himself to chemical engineering or physical chemistry, Haber traveled in the spring of 1894 to the [[Technical University of Karlsruhe]], without being certain of a position there. After having worked for several months at the university as an unpaid assistant, the professor of Chemical Technology, [[Hans Bunte]], put him on the payroll (on December 16, 1894). Two years later Haber made his ''Habilitation'' with a dissertation entitled ''Experimentelle Untersuchungen über Zersetzung und Verbrennung von Kohlenwasserstoffen'' (Experimental Studies on the Decomposition and Combustion of Hydrocarbons) (1896) and Haber received the title ''Privat-Dozent''. Bunte was especially interested in combustion chemistry and [[Carl Engler]], who was also in Karlsruhe, introduced Haber to the study of petroleum. Haber's subsequent work was greatly influenced by Bunte and Engler and he always spoke of them with great respect. In 1898, Haber published the textbook ''Grundriss der Technischen Elektrochemie auf theoretischer Gundlage'' (Outline of technical electrochemistry on theoretical basis) and obtained the honorary title of ''außerordentlicher'' (extraordinary) Professor in technical electrochemistry (December 6, 1898) that gave him tenure.
| | </math> |
| | | The midpoint ''A'' of ''FQ'' has coordinates (λ = ½): |
| In 1901, Haber married Clara Immerwahr, daughter of a respected Jewish family in Breslau, whom he had known as a teenager. Clara, who also had converted to the protestant religion, matched Fritz in ambition and determination, having fought against prejudice and opposition to become the first woman to obtain a doctorate in science at Breslau University. She committed suicide the night of May 1, 1915, shooting herself with Fritz’s army pistol, supposedly after heated arguments over Fritz’s involvement with the poison gas campaign on the western front during WWI.
| | :<math> |
| | | \frac{1}{2}\begin{pmatrix}0\\ f\end{pmatrix} + \frac{1}{2}\begin{pmatrix}x_1\\ -f\end{pmatrix} = |
| From 1904 on Haber worked on the catalytic formation of ammonia. In 1905 he published his book ''Thermodynamik technischer Gasreaktionen'' (Thermodynamics of technical gas reactions), which treats the foundations of his subsequent thermochemical work. In 1906 he succeeded [[Max Julius Le Blanc]] to the Karlsruhe chair of physical chemistry and electrochemistry. Le Blanc had left because he was appointed to the prestigious chair in Leipzig vacated by [[Wilhelm Ostwald]].
| | \begin{pmatrix}\frac{1}{2} x_1\\ 0\end{pmatrix}. |
| {{Image|Friz Haber group 1909.jpg|left|450px|<small>Research group of Fritz Haber in Karlsruhe (1909). Fritz Haber sits in the middle (5th from the left) just above man sitting on the ground; seated, 2nd from the left, R. Le Rossignol.</small>}} | | </math> |
| Haber remained in Karlsruhe until 1911 and gained a great reputation, especially in electrochemistry and chemical thermodynamics. He assembled a very large research group, of about forty persons (including research students), see the adjacent photograph.
| | Hence ''A'' lies on the ''x''-axis. |
| ====Ammonia synthesis====
| | The parabola has equation, |
| Haber's most important work during his latter years in Karlsruhe concerned the fixation of nitrogen. This work was mainly motivated by the needs of agriculture. It was known from the work of [[Justus von Liebig]] (1803–1873) that plants need nitrogen, but also that very few plants are able to grow on nitrogen from the air (through bacteria on their roots). Chili [[saltpetre]] (potassium and sodium nitrate) was the most important nitrogen-containing fertilizer at the end of the 19th century, but it was feared that the source would be exhausted somewhere early in the 20th century. Before Haber's work, ammonia was produced from coal and distributed as ammonium sulphate. Obviously an unlimited supply of nitrogen is present in the air (78% of the air is nitrogen), but the question was how to convert it to a form usable by plants. The reaction N<sub>2</sub> + H<sub>2</sub> → 2NH<sub>3</sub> is a good candidate for nitrogen (N<sub>2</sub>) fixation, because the resulting ammonia (NH<sub>3</sub>) can easily be converted into nitrate. The reaction is exothermic (releases heat), and therefore, is not energetically demanding. The problem is reaction speed, high [[pressure]] and a good [[catalyst]] are required to produce ammonia in finite amounts of times. Haber and his coworker, the young Briton Robert Le Rossignol, hit upon the idea of searching among [[transition metal]]s, because [[chromium]], [[manganese]], [[iron]] and [[nickel]] possess very definite catalytic properties. After a few years of hard work the two managed to synthesize ammonia in a way suitable for industrial upscaling. Despite their initial skepticism, in July 1909 representatives of [[BASF]] (Badische Anilin and Soda Fabrik) were finally convinced and enthusiastically began the search for the ideal catalyst and the construction of a synthetic ammonia factory which came on-stream in 1912. The industrial scale-up was largely directed by [[Carl Bosch]], a chemical engineer employed by BASF. The industrial synthesis is known as the [[Haber-Bosch process]]. Just before the outbreak of WWI, BASF was able to produce 30 ton ammonia daily, which turned out to be an important factor in prolonging the war, because the chemical route from ammonia to high explosives is a short one.
| | :<math> |
| ==Berlin==
| | y = \frac{1}{4f} x^2. |
| On October 1, 1911 Haber became director of the newly founded ''Kaiser-Wilhelm-Institut für physikalische Chemie und Elektrochemie'' ([[Kaiser Wilhelm Institute|KWI]]) in Dahlem-Berlin.<ref>Since 1953 the institute is called the ''Fritz-Haber-Institut der Max-Planck-Gesellschaft''</ref> With the function came a cross-appointment as honorary full professor at the university of Berlin. For Haber the appointment meant the end of his career as scientist and the beginning as science manager. For quite some time he was busy, not only with building and equipping the new laboratory, but also with the rules and regulations that would govern the new organization. At the festive opening of the institute in 1912, Emperor [[Wilhelm II]] expressed the wish that the new institute develop a sensor for explosive gases for use in coal mines. Haber set his section leader Richard Leider to work and together they came up with a kind of organ pipe (a ''Schlagwetterpfeife'', in English a "methane whistle") that gave an audible tone that was different for pure air than for air mixed with other gases. In agreement with the aim of the new institute, it was left to industry to manufacture and sell the new invention.<ref>Although the device worked, it was replaced soon by electric sensors of thermal conductivities of gas mixtures.</ref>
| | </math> |
| {{Image|2nd marriage Haber.jpg|right|350px|Son Hermann (b. 1902), Charlotte Nathan, and Fritz Haber (in captain's uniform) at wedding of Fritz and Charlotte, August 25, 1917. }} | | The equation of the tangent at ''P'' is |
| | | :<math> |
| Shortly after the outbreak of World War I in 1914, Fritz Haber undersigned gladly the extremely chauvinistic [[Manifesto of the 93]]. Haber and his institute were soon busy supporting the war effort. He assembled a large team, consisting at its peak of 2000 people with among them 150 chemists. Haber, who had left the army in 1890 as sergeant, was promoted to ''Hauptmann'' (captain)—but much to his chagrin never to a higher rank. It seems that Haber himself conceived of [[chlorine]] as a weapon. Supervised by Hauptmann Haber in person, the German army opened 6000 gas cylinders containing chlorine at [[Ypres]] on 22 April 1915. The gas drifted to trenches occupied by Algerian and French troops, who fled. The Germans failed to take advantage of this and for the rest of his life Haber claimed that Germany could have won the war had they then pressed on. In any case, this was the beginning of the application of chemical weapons on both sides, that, however, turned out not to be decisive for the outcome of the war. Haber's institute worked on improving gas masks and towards the end of the war invented [[mustard gas]] that was used at the western front in 1917.
| | y = y_1 + \frac{x_1}{2f} (x-x_1)\quad \hbox{with}\quad y_1 = \frac{x_1^2}{4f}. |
| | | </math> |
| Much to the fury of the allied countries, who saw Haber as a war criminal who had violated the [[Hague Conventions]] of 1899 and 1907, the Swedish Nobel committee awarded Haber alone (without Bosch or Le Rossignol) the chemistry Nobel Prize of 1918. During the ceremony the Prizes awarded during the war were also to be presented. Father and son [[Bragg]] (physics 1915), [[T. Richards]] (chemistry 1914) and [[J. Bordet]] (medicine 1919) boycotted the ceremonies because of Haber's presence.
| | This line intersects the ''x''-axis at ''y'' = 0, |
| | | :<math> |
| At the [[Treaty of Versailles|Piece Treaty of Versailles]] was decided that Germany had to pay the equivalent of 50 000 tons of gold. Remembering the estimate of [[Svante Arrhenius]] of 8 billion tons of gold in the sea, Haber sought a way of extracting gold from seawater. In 1920,
| | 0 = \frac{x_1^2}{4f} - \frac{x_1^2}{2f} + \frac{x_1}{2f} x |
| a group of experienced workers began to refine methods of analysis and economic extraction. In
| | \Longrightarrow \frac{x_1}{2f} x = \frac{x_1^2}{4f} \longrightarrow x = \tfrac{1}{2}x_1. |
| 1923, a ship was fitted with filtration plant and laboratory to sample the waters of the oceans. To their great astonishment and dismay they found only a small fraction of the expected amount of gold —the true content was only 1000th of the anticipated 5–10 mg per ton of sea
| | </math> |
| water. It was not until 1926 that Haber finally abandoned all hope of economic success.
| | The intersection of the tangent with the ''x''-axis is the point ''A'' = (½''x''<sub>1</sub>, 0) that lies on the midpoint of ''FQ''. The corresponding sides of the triangles ''FPA'' and ''QPA'' are of equal length and hence the triangles are congruent. |
| | |
| During the first fifteen years of the interbellum, Haber’s Institute became an internationally renowned center of research. In the years 1912–1933, more than 700 original papers were published. As many as half the members of staff were foreigners. In the spring of 1933 all Jewish personnel in the service of the German state (which included university professors and the staff of the KWI) were forced to resign. Although the (non-Jewish) physicist [[Max Planck]] pleaded with [[Adolf Hitler]] personally to exempt the war hero Fritz Haber, his plea was of no avail and Haber was forced to resign. He planned to accept a position in Palestine, but before being able to do that he died during visit to Basel (1934).
| |
| | |
| | |
| ==References==
| |
| *Daniel Charles, ''Master Mind: The Rise and Fall of Fritz Haber, the Nobel Laureate Who launched the Age of Chemical Warfare'', Ecco, New York (2005)
| |
| | |
| *Dietrich Stoltzenberg, ''Fritz Haber: Chemist, Nobel Laureate, German, Jew'', translated from the German by Charles Passage, Chemical Heritage Press, Philadelphia (2004)
| |
| | |
| *Margit Szöllösi-Janze, ''Fritz Haber: 1868-1934; eine Biographie'' Beck, München (1998)
| |
| | |
| *Patrick Coffey ''Cathedrals of Science: The Personalities and Rivalries That Made Modern Chemistry'', Oxford UP (2008)
| |
Parabolic mirror
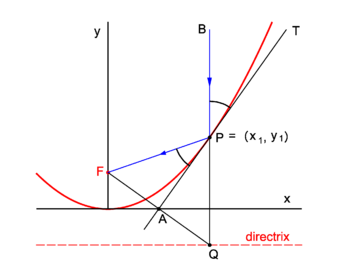
PD Image Fig. 2. Reflection in a parabolic mirror
Parabolic mirrors concentrate incoming vertical light beams in their focus. We show this.
Consider in figure 2 the arbitrary vertical light beam (blue, parallel to the y-axis) that enters the parabola and hits it at point P = (x1, y1). The parabola (red) has focus in point F. The incoming beam is reflected at P obeying the well-known law: incidence angle is angle of reflection. The angles involved are with the line APT which is tangent to the parabola at point P. It will be shown that the reflected beam passes through F.
Clearly ∠BPT = ∠QPA (they are vertically opposite angles). Further ∠APQ = ∠FPA because the triangles FPA and QPA are congruent and hence ∠FPA = ∠BPT.
We prove the congruence of the triangles: By the definition of the parabola the line segments FP and QP are of equal length, because the length of the latter segment is the distance of P to the directrix and the length of FP is the distance of P to the focus. The point F has the coordinates (0,f) and the point Q has the coordinates (x1, −f). The line segment FQ has the equation

The midpoint A of FQ has coordinates (λ = ½):

Hence A lies on the x-axis.
The parabola has equation,

The equation of the tangent at P is

This line intersects the x-axis at y = 0,

The intersection of the tangent with the x-axis is the point A = (½x1, 0) that lies on the midpoint of FQ. The corresponding sides of the triangles FPA and QPA are of equal length and hence the triangles are congruent.





