imported>Paul Wormer |
imported>Paul Wormer |
| (26 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| <!-- As an experiment I tried a Google translation of the German WP article. After a few hours of fixing it up I paused with it in the following state -->
| | ==Parabolic mirror== |
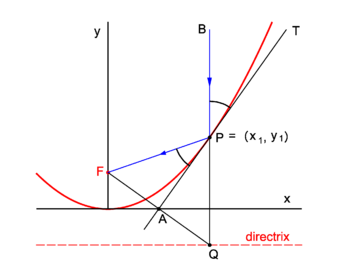
| | {{Image|Refl parab.png|right|350px|Fig. 2. Reflection in a parabolic mirror}} |
| | Parabolic mirrors concentrate incoming vertical light beams in their focus. We show this. |
|
| |
|
| [http://books.google.nl/books?id=0ekNIaJX3-YC&pg=PP2&dq=Fritz+Haber:+Chemist,+Nobel+Laureate,+German,+Jew:+A+Biography&cd=1#v=onepage&q=Fritz%20Haber%3A%20Chemist%2C%20Nobel%20Laureate%2C%20German%2C%20Jew%3A%20A%20Biography&f=false]
| | Consider in figure 2 the arbitrary vertical light beam (blue, parallel to the ''y''-axis) that enters the parabola and hits it at point ''P'' = (''x''<sub>1</sub>, ''y''<sub>1</sub>). The parabola (red) has focus in point ''F''. The incoming beam is reflected at ''P'' obeying the well-known law: incidence angle is angle of reflection. The angles involved are with the line ''APT'' which is tangent to the parabola at point ''P''. It will be shown that the reflected beam passes through ''F''. |
|
| |
|
| '''Fritz Haber''' (9 December 1868, [[Breslau]] – 29 January | | Clearly ∠''BPT'' = ∠''QPA'' (they are vertically opposite angles). Further ∠''APQ'' = ∠''FPA'' because the triangles ''FPA'' and ''QPA'' are congruent and hence ∠''FPA'' = ∠''BPT''. |
| 1934 [[Basel]]) was a German chemist and a pioneer of [[chemical warfare]]. Haber was awarded the [[Nobel Prize in Chemistry]] in 1918 for the synthesis of [[ammonia]] from the [[chemical element]]s [[hydrogen]] and [[nitrogen]].
| |
| == Life == | |
| Fritz Haber was born into a Jewish family. His father, Siegfried Haber, ran a business for dye pigments, paints, and pharmaceuticals. City councillor At Fritz's birth, serious complications occurred and his mother, Paula, died three weeks later. It seemed that Fritz's father blamed the child for the mother's death. This led in later life to tensions between father and son.
| |
|
| |
|
| <!--
| | We prove the congruence of the triangles: By the definition of the parabola the line segments ''FP'' and ''QP'' are of equal length, because the length of the latter segment is the distance of ''P'' to the directrix and the length of ''FP'' is the distance of ''P'' to the focus. The point ''F'' has the coordinates (0,''f'') and the point ''Q'' has the coordinates (''x''<sub>1</sub>, −''f''). The line segment ''FQ'' has the equation |
| Wife: Clara Immerwahr (chemist, b. 21-Jun-1870, m. 1901, d. 2-May-1915, suicide)
| | :<math> |
| Son: Hermann Haber (b. 1902, d. 1946, suicide)
| | \lambda\begin{pmatrix}0\\ f\end{pmatrix} + (1-\lambda)\begin{pmatrix}x_1\\ -f\end{pmatrix}, \quad 0\le\lambda\le 1. |
| Wife: Charlotte Nathan (m. 25-Aug-1917, div. 1927)
| | </math> |
| Daughter: Eva-Charlotte (b. 1918)
| | The midpoint ''A'' of ''FQ'' has coordinates (λ = ½): |
| Son: Ludwig-Fritz (b. 1921)
| | :<math> |
| | | \frac{1}{2}\begin{pmatrix}0\\ f\end{pmatrix} + \frac{1}{2}\begin{pmatrix}x_1\\ -f\end{pmatrix} = |
| High School: St. Elizabeth Classical School, Breslau, Prussia
| | \begin{pmatrix}\frac{1}{2} x_1\\ 0\end{pmatrix}. |
| University: University of Heidelberg (attended)
| | </math> |
| University: PhD Chemistry, University of Berlin (1891)
| | Hence ''A'' lies on the ''x''-axis. |
| Scholar: Chemistry, Swiss Federal Institute of Technology
| | The parabola has equation, |
| Scholar: Chemistry, University of Jena (1893-94)
| | :<math> |
| Teacher: Chemical Technology, University of Karlsruhe (1894-96)
| | y = \frac{1}{4f} x^2. |
| Lecturer: Chemical Technology, University of Karlsruhe (1896-1906)
| | </math> |
| Professor: Physical Chemistry and Electrochemistry, University of Karlsruhe (1906-11)
| | The equation of the tangent at ''P'' is |
| Professor: Kaiser Wilhelm Institute, Berlin-Dahlem (1911-33)
| | :<math> |
| Professor: Chemistry, University of Berlin (1911-33)
| | y = y_1 + \frac{x_1}{2f} (x-x_1)\quad \hbox{with}\quad y_1 = \frac{x_1^2}{4f}. |
| -->
| | </math> |
| | | This line intersects the ''x''-axis at ''y'' = 0, |
| Haber attended the humanistic gymnasium St. Elizabeth in Breslau, where he learned, besides the German language and literature, Latin and Greek and also mathematics and some physics, but hardly any chemistry. He had a keen interest in chemistry, as a school boy he already performed chemical experiments. Upon instigation of his father Haber first studied business, but in 1886 he switched to chemistry, first in Friedrich-Wilhelm's University [[Berlin]] under [[A.W. von Hoffmann]] and then in the summer semester of 1887 he moved to the [[University of Heidelberg]] under [[R. W. Bunsen]] and then . Haber received his doctorate in organic chemistry in May 1891 at the Friedrich Wilhelm University of Berlin for work performed under [[Carl Liebermann]] at the ''Technische Hochschule'' of [[Charlottenburg]] (now the [[Technical University Berlin]]) with a thesis entitled ''Über einige Derivate des Piperonal'' (On some derivatives of [[Piperonal]]). Liebermann was also professor at the Berlin university. Charlottenburg received right to grant doctorates only in 1899.
| | :<math> |
| | | 0 = \frac{x_1^2}{4f} - \frac{x_1^2}{2f} + \frac{x_1}{2f} x |
| In 1893, Haber converted to the [[Protestantism|Protestant]]-Christian faith against his father's wishes.
| | \Longrightarrow \frac{x_1}{2f} x = \frac{x_1^2}{4f} \longrightarrow x = \tfrac{1}{2}x_1. |
| | | </math> |
| After completing his University studies he voluntarily worked for a time in his father's business and, being interested in chemical technology, he also worked for a while under Professor [[Georg Lunge]] at the [[Eidgenössische Technische Hochschule Zürich|Institute of Technology]] at Zurich. He then finally decided to take up a scientific career and went for one and a half years to work with Ludwig Knorr at Jena, publishing with him a joint paper on diacetosuccinic ester. Still uncertain whether to devote himself to chemistry or physics, he accepted, an assistantship at the [[Technical University of Karlsruhe]] under the Professor of Chemical Technology there, [[Hans Bunte]]. He took his ''Habilitation'' in 1896 with the dissertation entitle ''Experimental Studies on the Decomposition of Hydrocarbons''. This hydrocarbon work had induced in him a liking for thermodynamics, which soon expanded into a liking for the then emerging area of physical chemistry. Haber's colleague and friend Hans Luggin, a former student of Svante Arrhenius, acted as catalyst. Haber rapidly metamorphosed into an extraordinary physical chemist who dominated and shaped the subject for the rest of his life.
| | The intersection of the tangent with the ''x''-axis is the point ''A'' = (½''x''<sub>1</sub>, 0) that lies on the midpoint of ''FQ''. The corresponding sides of the triangles ''FPA'' and ''QPA'' are of equal length and hence the triangles are congruent. |
| Bunte was especially interested in combustion chemistry and Carl Engler, who was also there, introduced Haber to the study of petroleum and Haber's subsequent work was greatly influenced by these two colleagues. Haber remained in Karlsruhe until 1911.
| |
| | |
| Two years later in 1898, Haber published the textbook "Fundamentals of practical electrochemistry"
| |
| in Karlsruhe and was appointed extraordinary [[professor]] of [[Chemical Technology]]. In 1906 he succeeded [[Max Le Blanc]] to the chair of Physical Chemistry and Electrochemistry in Karlsruhe.
| |
| | |
| From 1904 on Haber worked on the catalytic formation of ammonia. In 1905 he published his book "Thermodynamics of technical gas reactions", which treats the foundations of his subsequent thermo-chemical work. Haber applied on 13 October 1908 at the German Imperial Patent Office in Berlin for patent regarding a "method for synthetic preparation of ammonia from its elements" that was granted on the 8th of June 1911. Meanwhile, Haber had signed an employee contract with the [[BASF]] and you leave the patent to the economic recovery.<ref> Guenther
| |
| Luxbacher:''[http://www.wienerzeitung.at/Desktopdefault.aspx?TabID=3946&Alias=wzo&lexikon
| |
| = Science & letter = W & cob =] 5004 Bread and explosives.''In:''EXTRA
| |
| Lexikon, [[Wiener Zeitung ]].''</ ref>
| |
| | |
| As a result, he developed in 1909 together with [[Carl Bosch]] in the BASF, the [[Haber-Bosch process]], which was signed in 1910 for a patent. This procedure allowed for the synthetic production of ammonia as a substitute for [[saltpetre (chemical compound) | saltpetre]] to produce [[fertilizer | fertilizers]] and [[explosive]]. In 1911 Haber was appointed Director of
| |
| the [[Kaiser Wilhelm Institute]] for Physical Chemistry and [[Electrochemistry]] in Berlin-Dahlem and appointed 1912 to ordinary honorary professor of physical chemistry at the [[Humboldt University of Berlin | Berlin University]]. This institute is now designated as [[Fritz-Haber-Institut der Max-Planck-Society]] after him. Next is the Fritz Haber Center for Molecular Dynamics of the [[Hebrew University of Jerusalem | Hebrew University of Jerusalem]] named after
| |
| him. Because of its role as a military researcher and consultant, he was
| |
| assigned, previously deputy sergeant, the rank of [[Captain (officer)| captain]] granted. His experiments with [[phosgene]] and [[chlorine]] (a byproduct of the color production of the chemical industry), which - against the wishes of his first wife, [[Clara Immerwahr]] (Marriage
| |
| 1901), who held a PhD in chemistry - was started a few weeks after the war began, made him the father of [[poison gas] weapons], which were used in the [[First World War | World War I]] from Germany. A few days after the first German use of poison gas on 22 April 1915 at
| |
| [[Ypres]] committed suicide with his wife of Haber's service weapon. After the First World War he was due to the violation of the [[Hague Regulations]] from the [[Allied]] looking at times as a
| |
| [[war crimes]] and fled temporarily to the [[Switzerland]]. In his memoirs [[Otto Hahn reported]] on a conversation with Haber: "When I objected that this kind of warfare is contrary to the Hague Convention, he said that the French would have - albeit in poor shape, namely, gas-gun ammunition -- the beginning of this done. Too many lives are saved if the war could be completed more quickly in this way",<ref> Otto Hahn:''My Life.''Munich 1968. </ref>. From 1919, he tried vainly for six years to win from the sea [[gold]] in order to pay the [[German reparations]] too.
| |
| In April 1917 Haber had taken over the management of a ''technical committee'' pesticide, which was to deal with the disinfestation of accommodation (bed bugs and lice) and silos
| |
| (moth). This was done with [[hydrogen cyanide]] gas, which was
| |
| produced in the so-called''procedural''tun, was by [[sodium cyanide]] and [[potassium cyanide]] placed in an open wooden vat of dilute [[sulfuric acid]]. <ref> Jürgen Kalthoff:''The dealers of Zyklon B.''Hamburg 1998, ISBN 3-87975-713-5, p. 17-19. </ ref> In March 1919,
| |
| the [[German Society for Pest Control]] Founded (Degesch), whose first
| |
| line Haber, held in 1920 [[Walter Heerdt]].
| |
| | |
| [[Ferdinand Flury]], which was like Heerdt, and [[Bruno Tesch (chemist) | Bruno Tesch]] Haber's
| |
| former employee, developed a cyclone in 1920 and received patents for it. Cyclone A consisted of cyanide gas and the accompanying strong-smelling warning agent
| |
| [[bromoacetic acid]] <nowiki /> methyl ester,
| |
| which was delivered in bottles with a pressure
| |
| atomizer nozzle. A cyclone but could not
| |
| displace the vat method, and was considered
| |
| uneconomic. <ref> Jürgen Kalthoff:''The dealers
| |
| ...''. Hamburg 1998, ISBN
| |
| 3-87975-713-5, p. 28-30. </ ref> The decisive
| |
| progress towards a safe method bound with cyanide
| |
| in the warning agent to a porous carrier material
| |
| is not under pressure and after opening the tin
| |
| slowly releases gases, succeeded Walter Heerdt, of
| |
| this procedure on 20 June 1922 for
| |
| a patent for [[Zyklon B]] ((<ref> filed for patent
| |
| | country = U.S. | V-No = 438,818 | title =
| |
| procedures for pest control | A-Date = 1922-06-20
| |
| | date = 1926 V -12-27 | inventor = Walter Heerdt
| |
| | Applicant Degesch =)) </ ref>. This procedure was used
| |
| for fumigation with [[Zyklon B]]. <ref> Jürgen
| |
| Kalthoff:''The dealers ...'' Hamburg 1998, ISBN
| |
| 3-87975-713-5, p. 234 (often suggested a direct
| |
| connection Haber with Zyklon B is not given). </ref> Fritz Haber had since the founding of the
| |
| [[IG Farben]] 1925] in their [[Board].
| |
| | |
| After the [[Nazi]] 1933 at the Kaiser Wilhelm institutes the [[Aryan paragraph]]
| |
| s penetrated and dismissed the Jewish people, which even he could not prevent Haber in May 1933
| |
| could be put into retirement. He emigrated in the late fall of 1933 after the
| |
| [[Cambridge]], where he had not yet received a professorship at the [[University of Cambridge|University]] and died shortly after 1934 on his
| |
| way through [[Basel]].
| |
| == Impact == | |
| The research results show the
| |
| Haber [[Janus | Janus-faced]] of his scientific
| |
| work: On one hand, through the development of
| |
| ammonia synthesis (to manufacture explosive) or a
| |
| technical process for the production and use of
| |
| poison gas warfare, as it has become possible on
| |
| an industrial basis. Nor would it be without these
| |
| skills, the diet of mankind today is not
| |
| possible. The world
| |
| annual production of synthesized nitrogen
| |
| fertilizer is currently more than 100 million
| |
| tons.
| |
| Without this production makes possible the
| |
| Haber-Bosch process accounted for half of the
| |
| current world population, the food base. <ref>
| |
| Joerg Albrecht:''Bread and war from the
| |
| air.''In:''[[# Frankfurter Allgemeine Zeitung,
| |
| Frankfurter Allgemeine Zeitung Sunday (FAS) |
| |
| Frankfurter Allgemeine Zeitung Sunday ]].'' 41,
| |
| 2008, p. 77 (figures from 'Nature Geosience ").</ref>
| |
| | |
| == Literature == | |
| * Joerg Albrecht:''Bread and wars from the air. In the 77th'': [[# Frankfurter Allgemeine Zeitung, Frankfurter Allgemeine Sonntagszeitung (FAS) | Frankfurter Allgemeine
| |
| Zeitung Sunday]] 41/2008, p.
| |
| * [[Adolf Henning fruit]], Joachim Zepelin:''The tragedy of the despised love .''In: Mannheimer Forum''1994/95''. Piper, Munich 1995.
| |
| * Adolf Henning Frucht:''Fritz Haber and pest control during the 1st World War II and during the inflation''. In:''Dahlem Archive discussions''. Volume 11, 2005, p. 141-158.
| |
| * ((NDB | 7 | 386 | 389 | Haber, Fritz Jacob | Erna and Johannes Jaenicke))
| |
| * Fritz Richard Stern:''Five Germany and a life: memories''. Beck, Munich 2007, ISBN 978-3-406-55811-5.
| |
| * Dietrich Stoltzenberg:''Fritz Haber: Chemist, Nobel Laureate, German, Jew''. Wiley-VCH, Weinheim, 1998, ISBN 3-527-29573-9.
| |
| * Margit Szollosi-Janze:''Fritz Haber. 1868-1934. A Biography''. Beck, Munich 1998, ISBN -406-43548-3. Commonscat
| |