Modalities: Difference between revisions
imported>Mei Jin |
imported>Meg Taylor No edit summary |
||
| (16 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
In [[chromatography]], modality describes the different modes of target molecule transportation for separation. Modality differs by chromatography bed shapes and analyte (sample) mobile phase. | In [[chromatography]], modality describes the different modes of target molecule transportation for separation. Modality differs by chromatography bed shapes and analyte (sample) mobile phase. | ||
=='''Types by bed shape:''' == | |||
==='''Planar chromatography:'''=== | |||
'''''Method''''' | |||
'''Method''' | |||
Planar chromatography (PC) is a form of liquid chromatography in which the mixtures are eluted over a planar chromatographic bed to be separated <ref>(1)</ref>. The sample is applied as a spot to a marked position on a planar surface. After the evaporation of the solvent, the plate is placed in a chamber and immersed at one end in chosen solvent (mobile phase) <ref>(2)</ref>. The solvent then percolates through the porous media by capillary action, spreading the applied mixtures to different extents according to the characteristic of the component, which then can be visualized by staining or charring <ref>(1)</ref>. The substrate with greater affinity to the surface will travel more slowly and therefore move a shorter distance from the applied spot. | Planar chromatography (PC) is a form of liquid chromatography in which the mixtures are eluted over a planar chromatographic bed to be separated <ref>(1)</ref>. The sample is applied as a spot to a marked position on a planar surface. After the evaporation of the solvent, the plate is placed in a chamber and immersed at one end in chosen solvent (mobile phase) <ref>(2)</ref>. The solvent then percolates through the porous media by capillary action, spreading the applied mixtures to different extents according to the characteristic of the component, which then can be visualized by staining or charring <ref>(1)</ref>. The substrate with greater affinity to the surface will travel more slowly and therefore move a shorter distance from the applied spot. | ||
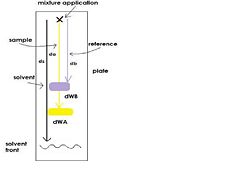
{{Image|imag_1.jpg|right|250px|Figure 1. A simple illustration of how a typical planar chromatography works. The movement of target molecule (to be separated out) is shown in yellow figure.}} | {{Image|imag_1.jpg|right|250px|Figure 1. A simple illustration of how a typical planar chromatography works. The movement of target molecule (to be separated out) is shown in yellow figure.}} | ||
'''''Types''''' | |||
'''Types''' | |||
There are two types of planar chromatography: Paper chromatography and Thin Layer Chromatography (TLC). They are similar methods; except in paper chromatography, cellulose paper coated with silicone oils, adsorbents (e.g., silica gel and alumina), or liquid ion-exchanger (stationary phase) is used for the separation. However, in TLC a sheet of glass, metal, or plastic coated with solid adsorbents in a thin, uniform layer is used. Due to the thinner spread, TLC requires smaller sample and gives greater resolution. | There are two types of planar chromatography: Paper chromatography and Thin Layer Chromatography (TLC). They are similar methods; except in paper chromatography, cellulose paper coated with silicone oils, adsorbents (e.g., silica gel and alumina), or liquid ion-exchanger (stationary phase) is used for the separation. However, in TLC a sheet of glass, metal, or plastic coated with solid adsorbents in a thin, uniform layer is used. Due to the thinner spread, TLC requires smaller sample and gives greater resolution. | ||
In Planar Chromatography, the retardation factor (R<sub>f</sub>) is the fraction of an analyte in the mobile phase of a chromatographic system. It refers to the migration relative to the solvent front and is related to the distribution coefficient of the compound <ref>(2)</ref>. | In Planar Chromatography, the retardation factor (R<sub>f</sub>) is the fraction of an analyte in the mobile phase of a chromatographic system. It refers to the migration relative to the solvent front and is related to the distribution coefficient of the compound <ref>(2)</ref>. | ||
| Line 23: | Line 17: | ||
R<sub>s</sub>=2(d<sub>b</sub>-d<sub>a</sub>)/(d<sub>W,A</sub>+d<sub>W,B</sub>) , | R<sub>s</sub>=2(d<sub>b</sub>-d<sub>a</sub>)/(d<sub>W,A</sub>+d<sub>W,B</sub>) , | ||
where d<sub>a</sub> and d<sub>b</sub> are the component distances of to the area of application and d<sub>WA</sub> and d<sub>WB</sub> are the width of the spots developed (Figure 1). | where d<sub>a</sub> and d<sub>b</sub> are the component distances of to the area of application and d<sub>WA</sub> and d<sub>WB</sub> are the width of the spots developed (Figure 1). | ||
In column chromatography, sample mixture is flown through a vertical glass column packed with the [[ | ==='''Column chromatography'''=== | ||
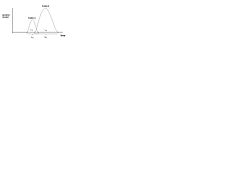
In column chromatography, sample mixture is flown through a vertical glass column packed with the [[adsorbent chemistries]]. The solvent and the non-targeted components (mobile phase) should be wash off quickly, whereas the target components would bind to the adsorbents and have to be eluded (Figure 2). The separation process can generally be described by Gaussian Curves (Figure 3). The retention time (t<sub>r</sub>) refers to the time of elution of peak maximum of the solute from the time of solute injection. WA and WB, the widths of Gaussian curves are elution time of each solute. | |||
{{Image|imag_2.jpg|right|250px|Figure 2: A simple diagram of a column chromatography}} | {{Image|imag_2.jpg|right|250px|Figure 2: A simple diagram of a column chromatography}} | ||
| Line 40: | Line 32: | ||
== '''Types by analyte mobile phase''' == | == '''Types by analyte mobile phase''' == | ||
== Liquid Chromatography == | ==='''Liquid Chromatography'''=== | ||
Liquid chromatography (LC) describes any chromatographic procedures in which the mobile phase is liquid. It includes thin layer chromatography (TLC), open chromatography, and high performance liquid chromatography (HPLC). | |||
Liquid chromatography (LC) describes any chromatographic procedures in which the mobile phase is liquid. It includes | TLC is a type of planar chromatography fed by capillary action as described above. | ||
Open chromatography is a simple column chromatography that is gravity fed. | |||
Open chromatography is a simple | |||
In theory, HPLC is much like open column, but it uses negative pressure created by pumping, and thereby expediting the process. Due to the high performance and high speed, HPLC gives higher resolution than other LC. But HPLC has many more components compared to regular open column chromatography. It has a pump that feeds the solvent into the sample, which together get injected into a column with packing materials by sample injector. A detector is attached to the opposite end of the column and sends the detected information to a data system for analysis (1) (see Figure 4). Therefore, HPLC columns are reusable and the sample detection and quantification can be achieved by the use of continuous flow detectors. HPLC is currently among the most favored chromatographic techniques (2). | In theory, HPLC is much like open column, but it uses negative pressure created by pumping, and thereby expediting the process. Due to the high performance and high speed, HPLC gives higher resolution than other LC. But HPLC has many more components compared to regular open column chromatography. It has a pump that feeds the solvent into the sample, which together get injected into a column with packing materials by sample injector. A detector is attached to the opposite end of the column and sends the detected information to a data system for analysis (1) (see Figure 4). Therefore, HPLC columns are reusable and the sample detection and quantification can be achieved by the use of continuous flow detectors. HPLC is currently among the most favored chromatographic techniques (2). | ||
There are several modes of separation available in HPLC; adsorption, bound phase, ion exchange, ion pair partition, gel permeation-gel exclusion, affinity, etc. HPLC performance can be evaluated using many parameters, like peak asymmetry, selectivity, resolution, and pressure drop across the column. | There are several modes of separation available in HPLC; adsorption, bound phase, ion exchange, ion pair partition, gel permeation-gel exclusion, affinity, etc. HPLC performance can be evaluated using many parameters, like peak asymmetry, selectivity, resolution, and pressure drop across the column. | ||
{{Image|imag_4.jpg|right|250px|Figure 4: An illustration of how HPLC works}} | {{Image|imag_4.jpg|right|250px|Figure 4: An illustration of how HPLC works}} | ||
'''Method''' | ==='''Gas Chromatography '''=== | ||
GC describes all chromatographic methods in which the mobile phase is a gas. The sample is usually introduced into the chromatograph through the sample inlet into a continuous flow of mobile phase. The sample is then vaporized in the inlet system and transported by the carrier gas to the thermostatted column, where separation occurs. The individual components produce an electrical signal in the detector, which may have provision for the inlet of additional make-up gas (Figure 5). This is necessary to permit separate optimization of gas flow through the column and detector. After the detector signal is amplified and conducted to a recording device, the sample components can be identified from their characteristic retention times (2). | '''''Method''''' | ||
GC describes all chromatographic methods in which the mobile phase is a gas. The sample is usually introduced into the chromatograph through the sample inlet into a continuous flow of mobile phase. The sample is then vaporized in the inlet system and transported by the carrier gas to the thermostatted column, where separation occurs. The individual components produce an electrical signal in the detector, which may have provision for the inlet of additional make-up gas (Figure 5). This is necessary to permit separate optimization of gas flow through the column and detector. After the detector signal is amplified and conducted to a recording device, the sample components can be identified from their characteristic retention times <ref>(2)</ref>. | |||
{{Image|imag_5.jpg|right|250px|Figure 5: An illustration of gas chromatography}} | {{Image|imag_5.jpg|right|250px|Figure 5: An illustration of gas chromatography}} | ||
'''Types''' | '''''Types''''' | ||
There are two types of GC; gas-solid chromatography (GSC) that uses solid adsorbents for separation and gas-liquid chromatography (GLC) which uses liquid coated on a solid packed column or deposited directly on the column walls. The main adsorbents for GSC are silica, charcoal, alumina or molecular sieves. For GLC, polymers based on silicon-oxygen-silicon backbone are mostly commonly used for adsorbents. | There are two types of GC; gas-solid chromatography (GSC) that uses solid adsorbents for separation and gas-liquid chromatography (GLC) which uses liquid coated on a solid packed column or deposited directly on the column walls. The main adsorbents for GSC are silica, charcoal, alumina or molecular sieves. For GLC, polymers based on silicon-oxygen-silicon backbone are mostly commonly used for adsorbents. | ||
Advantages/Disadvantages | Advantages/Disadvantages | ||
GSC has several disadvantages; retention times are long and adsorbents are difficult to standardize, as well as easy to catalyze. But it also exhibits some advantages over GLC. It has greater selectivity and adsorbents are stable over a wide temperature range, allowing high column temperatures (2). | GSC has several disadvantages; retention times are long and adsorbents are difficult to standardize, as well as easy to catalyze. But it also exhibits some advantages over GLC. It has greater selectivity and adsorbents are stable over a wide temperature range, allowing high column temperatures <ref>(2)</ref>. | ||
==References== | ==References== | ||
(1) Robards, K., Paul R. Haddad, and Peter E. Jackson. Principles and Practice of Modern Chromatographic Methods. London: Academic, 1994. Print. | |||
(2) Braithwaite, A., and F. J. Smith. Chromatographic Methods. London: Blackie Academic & Professional, 1996. Print. | (2) Braithwaite, A., and F. J. Smith. Chromatographic Methods. London: Blackie Academic & Professional, 1996. Print. | ||
{{reflist}} | |||
Latest revision as of 03:16, 7 October 2013
In chromatography, modality describes the different modes of target molecule transportation for separation. Modality differs by chromatography bed shapes and analyte (sample) mobile phase.
Types by bed shape:
Planar chromatography:
Method Planar chromatography (PC) is a form of liquid chromatography in which the mixtures are eluted over a planar chromatographic bed to be separated [1]. The sample is applied as a spot to a marked position on a planar surface. After the evaporation of the solvent, the plate is placed in a chamber and immersed at one end in chosen solvent (mobile phase) [2]. The solvent then percolates through the porous media by capillary action, spreading the applied mixtures to different extents according to the characteristic of the component, which then can be visualized by staining or charring [3]. The substrate with greater affinity to the surface will travel more slowly and therefore move a shorter distance from the applied spot.
Types There are two types of planar chromatography: Paper chromatography and Thin Layer Chromatography (TLC). They are similar methods; except in paper chromatography, cellulose paper coated with silicone oils, adsorbents (e.g., silica gel and alumina), or liquid ion-exchanger (stationary phase) is used for the separation. However, in TLC a sheet of glass, metal, or plastic coated with solid adsorbents in a thin, uniform layer is used. Due to the thinner spread, TLC requires smaller sample and gives greater resolution. In Planar Chromatography, the retardation factor (Rf) is the fraction of an analyte in the mobile phase of a chromatographic system. It refers to the migration relative to the solvent front and is related to the distribution coefficient of the compound [4]. Rf=(Distance travelled by solute)/(Distance travelled by solvent front) The resolution (Rs) is a measure of how well two species are separated. Rs between an adjacent pair of spots can be expressed in terms of distances travelled by the solute and solvent, Rs=2(db-da)/(dW,A+dW,B) , where da and db are the component distances of to the area of application and dWA and dWB are the width of the spots developed (Figure 1).
Column chromatography
In column chromatography, sample mixture is flown through a vertical glass column packed with the adsorbent chemistries. The solvent and the non-targeted components (mobile phase) should be wash off quickly, whereas the target components would bind to the adsorbents and have to be eluded (Figure 2). The separation process can generally be described by Gaussian Curves (Figure 3). The retention time (tr) refers to the time of elution of peak maximum of the solute from the time of solute injection. WA and WB, the widths of Gaussian curves are elution time of each solute.
The resolution, Rs can be characterized using the following equation,
Rs=2(tr,B-tr,A)/(WB+WA ) ,
where tr,B and tr,A are the retention time of solute A and B; and WB and WA are the width of Gaussian curves as shown in Figure 3.
Types by analyte mobile phase
Liquid Chromatography
Liquid chromatography (LC) describes any chromatographic procedures in which the mobile phase is liquid. It includes thin layer chromatography (TLC), open chromatography, and high performance liquid chromatography (HPLC). TLC is a type of planar chromatography fed by capillary action as described above. Open chromatography is a simple column chromatography that is gravity fed. In theory, HPLC is much like open column, but it uses negative pressure created by pumping, and thereby expediting the process. Due to the high performance and high speed, HPLC gives higher resolution than other LC. But HPLC has many more components compared to regular open column chromatography. It has a pump that feeds the solvent into the sample, which together get injected into a column with packing materials by sample injector. A detector is attached to the opposite end of the column and sends the detected information to a data system for analysis (1) (see Figure 4). Therefore, HPLC columns are reusable and the sample detection and quantification can be achieved by the use of continuous flow detectors. HPLC is currently among the most favored chromatographic techniques (2). There are several modes of separation available in HPLC; adsorption, bound phase, ion exchange, ion pair partition, gel permeation-gel exclusion, affinity, etc. HPLC performance can be evaluated using many parameters, like peak asymmetry, selectivity, resolution, and pressure drop across the column.
Gas Chromatography
Method GC describes all chromatographic methods in which the mobile phase is a gas. The sample is usually introduced into the chromatograph through the sample inlet into a continuous flow of mobile phase. The sample is then vaporized in the inlet system and transported by the carrier gas to the thermostatted column, where separation occurs. The individual components produce an electrical signal in the detector, which may have provision for the inlet of additional make-up gas (Figure 5). This is necessary to permit separate optimization of gas flow through the column and detector. After the detector signal is amplified and conducted to a recording device, the sample components can be identified from their characteristic retention times [5].
Types There are two types of GC; gas-solid chromatography (GSC) that uses solid adsorbents for separation and gas-liquid chromatography (GLC) which uses liquid coated on a solid packed column or deposited directly on the column walls. The main adsorbents for GSC are silica, charcoal, alumina or molecular sieves. For GLC, polymers based on silicon-oxygen-silicon backbone are mostly commonly used for adsorbents. Advantages/Disadvantages GSC has several disadvantages; retention times are long and adsorbents are difficult to standardize, as well as easy to catalyze. But it also exhibits some advantages over GLC. It has greater selectivity and adsorbents are stable over a wide temperature range, allowing high column temperatures [6].
References
(1) Robards, K., Paul R. Haddad, and Peter E. Jackson. Principles and Practice of Modern Chromatographic Methods. London: Academic, 1994. Print. (2) Braithwaite, A., and F. J. Smith. Chromatographic Methods. London: Blackie Academic & Professional, 1996. Print.