imported>Milton Beychok |
|
| Line 1: |
Line 1: |
| '''Petroleum crude oil''' is a naturally occurring, [[flammability|flammable]] liquid found primarily in underground rock formations and consists of a complex mixture of [[hydrocarbon]]s of various [[molecular weight]]s plus other [[organic compound]]s.
| | {{AccountNotLive}} |
| | __NOTOC__ |
| | [[File:Crude oil-fired power plant.jpg|thumb|right|225px|Industrial air pollution source]] |
| | Atmospheric dispersion modeling is the mathematical simulation of how air pollutants disperse in the ambient atmosphere. It is performed with computer programs that solve the mathematical equations and algorithms which simulate the pollutant dispersion. The dispersion models are used to estimate or to predict the downwind concentration of air pollutants emitted from sources such as industrial plants, vehicular traffic or accidental chemical releases. |
|
| |
|
| The [[Latin]] word '''''petroleum''''' was first used to describe petroleum crude oil by the [[Germany|German]] mineralogist [[Georg Bauer]] (also known as Georgius Agricola) in the treatise ''De Natura Fossilium'', published in 1546<ref>{{cite book |author=Bauer Georg|title=De Natura Fossilium |year=1546}} Translated in 1955 by Mark C. Bandy and Jean A. Bandy </ref> The [[Greek language|Greek]] word for petroleum is ''πετρέλαιον'', meaning "rock oil". | | Such models are important to governmental agencies tasked with protecting and managing the ambient air quality. The models are typically employed to determine whether existing or proposed new industrial facilities are or will be in compliance with the National Ambient Air Quality Standards (NAAQS) in the United States or similar regulations in other nations. The models also serve to assist in the design of effective control strategies to reduce emissions of harmful air pollutants. During the late 1960's, the Air Pollution Control Office of the U.S. Environmental Protection Agency (U.S. EPA) initiated research projects to develop models for use by urban and transportation planners.<ref>J.C. Fensterstock et al, "Reduction of air pollution potential through environmental planning", ''JAPCA'', Vol. 21, No. 7, 1971.</ref> |
|
| |
|
| == Composition ==
| | Air dispersion models are also used by emergency management personnel to develop emergency plans for accidental chemical releases. The results of dispersion modeling, using worst case accidental releases and meteorological conditions, can provide estimated locations of impacted areas and be used to determine appropriate protective actions. At industrial facilities in the United States, this type of consequence assessment or emergency planning is required under the Clean Air Act (CAA) codified in Part 68 of Title 40 of the Code of Federal Regulations. |
|
| |
|
| Both crude oil and [[natural gas]] are predominantly mixtures of [[hydrocarbons]]. At typical ambient conditions of pressure and temperature, the lower molecular weight hydrocarbons [[methane]], [[ethane]], [[propane]] and [[butane]] occur as gases, while the higher molecular weight ones ([[pentane]] and higher) are in the form of liquids or solids. However, in the underground [[oil reservoir]]s the proportion which is gas or liquid varies depending on the subsurface conditions, and on the [[phase diagram]] of the petroleum mixture.<ref name="Hyne 2001">
| | The dispersion models vary depending on the mathematics used to develop the model, but all require the input of data that may include: |
| {{Cite book
| |
| | last = Hyne
| |
| | first = Norman J.
| |
| | title = Nontechnical Guide to Petroleum Geology, Exploration, Drilling, and Production
| |
| | date = 2001
| |
| | publisher = PennWell Corporation
| |
| | isbn = 087814823X
| |
| | pages = 1-4}} </ref>
| |
|
| |
|
| An [[oil well]] produces predominantly crude oil, with some natural gas [[solubility|dissolved]] in it. Because the pressure is lower at the surface than underground, some of the gas will come out of [[solution]] and be recovered as '''associated gas''' or '''solution gas'''. A [[gas well]] produces predominately natural gas. However, because the underground temperature and pressure are higher than at the surface, the gas may contain heavier hydrocarbons such as [[pentane]], [[hexane]], and [[heptane]] in the [[gaseous state]]. Under surface conditions these will [[condense]] out of the gas and form [[natural gas condensate]], often shortened to '''condensate'''. Condensate resembles [[gasoline]] in appearance and is similar in composition to some [[volatility (chemistry)|volatile]] [[light crude oil]]s.
| | * Meteorological conditions such as wind speed and direction, the amount of atmospheric turbulence (as characterized by what is called the "stability class"), the ambient air temperature, the height to the bottom of any inversion aloft that may be present, cloud cover and solar radiation. |
| | * The emission parameters such the type of source (i.e., point, line or area), the mass flow rate, the source location and height, the source exit velocity, and the source exit temperature. |
| | * Terrain elevations at the source location and at receptor locations, such as nearby homes, schools, businesses and hospitals. |
| | * The location, height and width of any obstructions (such as buildings or other structures) in the path of the emitted gaseous plume as well as the terrain surface roughness (which may be characterized by the more generic parameters "rural" or "city" terrain). |
|
| |
|
| The proportion of hydrocarbons in the petroleum mixture is highly variable between different [[oil fields]] and ranges from as much as 97% by weight in the lighter oils to as little as 50% in the heavier oils and [[bitumen]]s.
| | Many of the modern, advanced dispersion modeling programs include a pre-processor module for the input of meteorological and other data, and many also include a post-processor module for graphing the output data and/or plotting the area impacted by the air pollutants on maps. The plots of areas impacted usually include isopleths showing areas of pollutant concentrations that define areas of the highest health risk. The isopleths plots are useful in determining protective actions for the public and first responders. |
|
| |
|
| The hydrocarbons in crude oil are mostly [[alkane]]s, [[cycloalkane]]s and various [[aromatic hydrocarbon]]s while the other organic compounds contain [[nitrogen]], [[oxygen]] and [[sulfur]], and trace amounts of metals such as [[iron]], [[nickel]], [[copper]] and [[vanadium]]. The exact molecular composition varies widely from formation to formation but the proportion of [[chemical element]]s vary over fairly narrow limits as follows:<ref name="Speight"> {{Cite book | | The atmospheric dispersion models are also known as atmospheric diffusion models, air dispersion models, air quality models, and air pollution dispersion models. |
| | last = Speight
| |
| | first = James G.
| |
| | title = The Chemistry and Technology of Petroleum
| |
| | date = 1999
| |
| | publisher = Marcel Dekker
| |
| | isbn = 0824702174
| |
| | pages = 215–216
| |
| }}
| |
| </ref>
| |
|
| |
|
| {| class = "wikitable"
| | ==Atmospheric layers== |
| |+ Elements by weight
| |
| |-
| |
| ! Element !! Percent range
| |
| |-
| |
| |Carbon || 83 to 87%
| |
| |-
| |
| |Hydrogen || 10 to 14%
| |
| |-
| |
| |Nitrogen || 0.1 to 2%
| |
| |-
| |
| |Oxygen || 0.1 to 1.5%
| |
| |-
| |
| |Sulfur || 0.5 to 6%
| |
| |-
| |
| |Metals || less than 1000 ppm
| |
| |}
| |
| | |
| Four different types of hydrocarbon molecules appear in crude oil. The relative percentage of each varies from oil to oil, determining the properties of each oil.<ref name="Hyne 2001" />
| |
|
| |
|
| {| class = "wikitable"
| | Discussion of the layers in the Earth's atmosphere is needed to understand where airborne pollutants disperse in the atmosphere. The layer closest to the Earth's surface is known as the ''troposphere''. It extends from sea-level up to a height of about 18 km and contains about 80 percent of the mass of the overall atmosphere. The ''stratosphere'' is the next layer and extends from 18 km up to about 50 km. The third layer is the ''mesosphere'' which extends from 50 km up to about 80 km. There are other layers above 80 km, but they are insignificant with respect to atmospheric dispersion modeling. |
| |+ Hydrocarbon types by weight
| |
| |-
| |
| ! Hydrocarbon !! Average !! Range
| |
| |-
| |
| |[[Paraffin]]s || 30% || 15 to 60%
| |
| |-
| |
| |[[Naphthene]]s || 49% || 30 to 60%
| |
| |-
| |
| |[[Aromatic]]s || 15% || 3 to 30%
| |
| |-
| |
| |[[Asphalt]]ics || 6% || remainder
| |
| |-
| |
| |}
| |
| | |
| [[Image:Total World Oil Reserves.PNG|thumb|250px|Most of the world's oils are non-conventional.<ref>{{cite paper | author = Alboudwarej et al. | title = Highlighting Heavy Oil
| |
| | publisher = Oilfield Review
| |
| | date = Summer 2006
| |
| | url = http://www.slb.com/media/services/resources/oilfieldreview/ors06/sum06/heavy_oil.pdf
| |
| | format = PDF
| |
| | accessdate = 2008-05-24}}</ref>]]
| |
| Crude oil varies greatly in appearance depending on its composition. It is usually black or dark brown (although it may be yellowish or even greenish). In the reservoir it is usually found in association with [[natural gas]], which being lighter forms a gas cap over the petroleum, and [[saline water]] which, being heavier than most forms of crude oil, generally sinks beneath it. Crude oil may also be found in semi-solid form mixed with sand and water, as in the [[Athabasca oil sands]] in [[Canada]], where it is usually referred to as crude [[bitumen]]. In Canada, bitumen is considered a sticky, tar-like form of crude oil which is so thick and heavy that it must be heated or diluted before it will flow.<ref>
| |
| {{cite web
| |
| | title = Oil Sands - Glossary
| |
| | work = Mines and Minerals Act
| |
| | publisher = Government of Alberta
| |
| | date = 2007
| |
| | url = http://www.energy.gov.ab.ca/OilSands/1106.asp
| |
| | accessdate = 2008-10-02}}
| |
| </ref> Venezuela also has large amounts of oil in the [[Orinoco oil sands]], although the hydrocarbons trapped in them are more fluid than in Canada and are usually called [[extra heavy oil]]. These oil sands resources are called [[unconventional oil]] to distinguish them from oil which can be extracted using traditional oil well methods. Between them, Canada and [[Venezuela]] contain an estimated {{convert|3.6|Toilbbl}} of bitumen and extra-heavy oil, about twice the volume of the world's reserves of conventional oil.<ref>
| |
| {{cite web
| |
| | title = Oil Sands in Canada and Venezuela
| |
| | publisher = Infomine Inc.
| |
| | date = 2008
| |
| | url = http://oilsands.infomine.com/countries/
| |
| | accessdate = 2008-10-02}}
| |
| </ref>
| |
|
| |
|
| Petroleum is used mostly, by volume, for producing [[fuel oil]] and [[gasoline]] ([[petrol]]), both important ''"[[primary energy]]"'' sources.<ref>[http://www.iea.org/bookshop/add.aspx?id=144 IEA Key World Energy Statistics]</ref>
| | The lowest part of the troposphere is called the ''atmospheric boundary layer (ABL)'' or the ''planetary boundary layer (PBL)'' and extends from the Earth's surface up to about 1.5 to 2.0 km in height. The air temperature of the atmospheric boundary layer decreases with increasing altitude until it reaches what is called the ''inversion layer'' (where the temperature increases with increasing altitude) that caps the atmospheric boundary layer. The upper part of the troposphere (i.e., above the inversion layer) is called the ''free troposphere'' and it extends up to the 18 km height of the troposphere. |
| 84% by volume of the hydrocarbons present in petroleum is converted into energy-rich fuels (petroleum-based fuels), including gasoline, diesel, jet, heating, and other fuel oils, and [[liquefied petroleum gas]].<ref>[http://www.eia.doe.gov/kids/energyfacts/sources/non-renewable/oil.html#Howused "Crude oil is made into different fuels"]</ref> The lighter grades of crude oil produce the best yields of these products, but as the world's reserves of light and medium oil are depleted, [[oil refineries]] are increasingly having to process heavy oil and bitumen, and use more complex and expensive methods to produce the products required. Because heavier crude oils have too much carbon and not enough hydrogen, these processes generally involve removing carbon from or adding hydrogen to the molecules, and using [[fluid catalytic cracking]] to convert the longer, more complex molecules in the oil to the shorter, simpler ones in the fuels.
| |
|
| |
|
| Due to its high [[energy density]], easy [[transport]]ability and [[oil reserves|relative abundance]], oil has become the world's most important source of energy since the mid-1950s. Petroleum is also the raw material for many [[chemical]] products, including [[pharmaceutical]]s, [[solvent]]s, [[fertilizer]]s, [[pesticide]]s, and [[plastic]]s; the 16% not used for energy production is converted into these other materials.
| | The ABL is the most important layer with respect to the emission, transport and dispersion of airborne pollutants. The part of the ABL between the Earth's surface and the bottom of the inversion layer is known as the ''mixing layer''. Almost all of the airborne pollutants emitted into the ambient atmosphere are transported and dispersed within the mixing layer. Some of the emissions penetrate the inversion layer and enter the free troposphere above the ABL. |
|
| |
|
| Petroleum is found in [[porosity|porous]] [[rock formations]] in the upper [[stratum|strata]] of some areas of the [[Earth]]'s [[crust (geology)|crust]]. There is also petroleum in [[tar sands|oil sands (tar sands)]]. Known [[oil reserves|reserves of petroleum]] are typically estimated at around 190 km<sup>3</sup> (1.2 [[1000000000 (number)|trillion]] [[long and short scales|(short scale)]] [[barrel (unit)|barrels]]) without oil sands,<ref>[http://www.eia.doe.gov/emeu/international/reserves.html EIA reserves estimates]</ref> or 595 km<sup>3</sup> (3.74 trillion barrels) with oil sands.<ref> [http://www.cera.com/aspx/cda/public1/news/pressReleases/pressReleaseDetails.aspx?CID=8444 CERA report on total world oil]</ref> Consumption is currently around {{convert|84|Moilbbl}} per day, or 4.9 km<sup>3</sup> per year. Because the [[EROEI|energy return over energy invested (EROEI)]] ratio of oil is constantly falling (due to physical phenomena such as residual oil saturation, and the economic factor of rising marginal extraction costs), recoverable oil reserves are significantly less than total oil in place{{Citation needed|date=August 2009}} . At current consumption levels, and assuming that oil will be consumed only from reservoirs, known recoverable reserves would be gone around 2039{{Citation needed|date=August 2009}} , potentially leading to a global [[energy crisis]]. However, to date discoveries of new oil reserves have more than matched increased usage. In addition, there are factors which may extend or reduce this estimate, including the increasing demand for petroleum in developing nations, particularly [[China]] and [[India]]; further new discoveries; increased economic viability of recoveries from more difficult to exploit sources; [[Mitigation of peak oil|energy conservation and use of alternative energy sources]]; and new economically viable exploitation of unconventional oil sources.
| | In summary, the layers of the Earth's atmosphere from the surface of the ground upwards are: the ABL made up of the mixing layer capped by the inversion layer; the free troposphere; the stratosphere; the mesosphere and others. Many atmospheric dispersion models are referred to as ''boundary layer models'' because they mainly model air pollutant dispersion within the ABL. To avoid confusion, models referred to as ''mesoscale models'' have dispersion modeling capabilities that can extend horizontally as much as a few hundred kilometres. It does not mean that they model dispersion in the mesosphere. |
|
| |
|
| ==Chemistry== | | ==Gaussian air pollutant dispersion equation== |
| [[Image:Octane molecule 3D model.png|thumb|right|250px|[[Octane]], a [[hydrocarbon]] found in petroleum, lines are [[single bond]]s, black [[spheres]] are [[carbon]], white spheres are [[hydrogen]]]]
| |
| Petroleum is a mixture of a very large number of different [[hydrocarbon]]s; the most commonly found molecules are [[alkane]]s (linear or branched), [[cycloalkane]]s, [[aromatic hydrocarbon]]s, or more complicated chemicals like [[asphaltene]]s. Each petroleum variety has a unique mix of [[molecule]]s, which define its physical and chemical properties, like color and [[viscosity]].
| |
|
| |
|
| The '''alkanes''', also known as '''paraffins''', are [[saturation (chemistry)|saturated]] hydrocarbons with straight or branched chains which contain only [[carbon]] and [[hydrogen]] and have the general formula '''C<sub>n</sub>H<sub>2n+2</sub>''' They generally have from 5 to 40 carbon atoms per molecule, although trace amounts of shorter or longer molecules may be present in the mixture. | | The technical literature on air pollution dispersion is quite extensive and dates back to the 1930s and earlier. One of the early air pollutant plume dispersion equations was derived by Bosanquet and Pearson.<ref>C.H. Bosanquet and J.L. Pearson, "The spread of smoke and gases from chimneys", ''Trans. Faraday Soc.'', 32:1249, 1936.</ref> Their equation did not assume Gaussian distribution nor did it include the effect of ground reflection of the pollutant plume. |
|
| |
|
| The alkanes from [[pentane]] (C<sub>5</sub>H<sub>12</sub>) to [[octane]] (C<sub>8</sub>H<sub>18</sub>) are [[oil refinery|refined]] into [[gasoline]] (petrol), the ones from [[nonane]] (C<sub>9</sub>H<sub>20</sub>) to [[hexadecane]] (C<sub>16</sub>H<sub>34</sub>) into [[diesel fuel]] and [[kerosene]] (primary component of many types of [[jet fuel]]), and the ones from hexadecane upwards into [[fuel oil]] and [[lubricating oil]]. At the heavier end of the range, [[paraffin wax]] is an alkane with approximately 25 carbon atoms, while [[asphalt]] has 35 and up, although these are usually [[Fluid catalytic cracking|cracked]] by modern refineries into more valuable products. The shortest molecules, those with four or fewer carbon atoms, are in a gaseous state at room temperature. They are the petroleum gases. Depending on demand and the cost of recovery, these gases are either flared off, sold as liquified petroleum gas under pressure, or used to power the refinery's own burners. During the winter, Butane (C<sub>4</sub>H<sub>10</sub>), is blended into the gasoline pool at high rates, because butane's high vapor pressure assists with cold starts. Liquified under pressure slightly above atmospheric, it is best known for powering cigarette lighters, but it is also a main fuel source for many developing countries. Propane can be liquified under modest pressure, and is consumed for just about every application relying on petroleum for energy, from cooking to heating to transportation.
| | Sir Graham Sutton derived an air pollutant plume dispersion equation in 1947<ref>O.G. Sutton, "The problem of diffusion in the lower atmosphere", ''QJRMS'', 73:257, 1947.</ref><ref>O.G. Sutton, "The theoretical distribution of airborne pollution from factory chimneys", ''QJRMS'', 73:426, 1947.</ref> which did include the assumption of Gaussian distribution for the vertical and crosswind dispersion of the plume and also included the effect of ground reflection of the plume. |
|
| |
|
| The '''cycloalkanes''', also known as '''naphthenes''', are saturated hydrocarbons which have one or more carbon rings to which hydrogen atoms are attached according to the formula '''C<sub>n</sub>H<sub>2n</sub>'''. Cycloalkanes have similar properties to alkanes but have higher boiling points. | | Under the stimulus provided by the advent of stringent environmental control regulations, there was an immense growth in the use of air pollutant plume dispersion calculations between the late 1960s and today. A great many computer programs for calculating the dispersion of air pollutant emissions were developed during that period of time and they were commonly called "air dispersion models". The basis for most of those models was the '''Complete Equation For Gaussian Dispersion Modeling Of Continuous, Buoyant Air Pollution Plumes''' shown below:<ref name=Beychok>{{cite book|author=M.R. Beychok|title=Fundamentals Of Stack Gas Dispersion|edition=4th Edition| publisher=author-published|year=2005|isbn=0-9644588-0-2}}.</ref><ref>{{cite book|author=D. B. Turner| title=Workbook of atmospheric dispersion estimates: an introduction to dispersion modeling| edition=2nd Edition |publisher=CRC Press|year=1994|isbn=1-56670-023-X}}.</ref> |
|
| |
|
| The '''aromatic hydrocarbons''' are [[degree of unsaturation|unsaturated hydrocarbons]] which have one or more planar six-carbon rings called [[benzene ring]]s, to which hydrogen atoms are attached with the formula '''C<sub>n</sub>H<sub>n</sub>'''. They tend to burn with a sooty flame, and many have a sweet aroma. Some are [[carcinogenic]].
| |
|
| |
|
| These different molecules are separated by [[fractional distillation]] at an oil refinery to produce gasoline, jet fuel, kerosene, and other hydrocarbons. For example [[2,2,4-trimethylpentane]] (isooctane), widely used in [[gasoline]], has a chemical formula of C<sub>8</sub>H<sub>18</sub> and it reacts with oxygen [[exothermic]]ally:<ref>[http://www.webmo.net/curriculum/heat_of_combustion/heat_of_combustion_key.html Heat of Combustion of Fuels]</ref>
| | <math>C = \frac{\;Q}{u}\cdot\frac{\;f}{\sigma_y\sqrt{2\pi}}\;\cdot\frac{\;g_1 + g_2 + g_3}{\sigma_z\sqrt{2\pi}}</math> |
|
| |
|
| <!-- Alternate way to display
| | {| border="0" cellpadding="2" |
| C<sub>8</sub>H<sub>18</sub>(''l'') + 12.5O<sub>2</sub>(''g'') → 8CO<sub>2</sub>(''g'') + 9H<sub>2</sub>O(''g'') + 5.43 MJ/mol (of octane)
| |
| -->
| |
| <math>2\mathrm{C}_8 \mathrm{H}_{18(l)} + 25\mathrm{O}_{2(g)} \rightarrow \; 16\mathrm{CO}_{2(g)} + 18\mathrm{H}_2 \mathrm{O}_{(l)} + 10.86 \ \mathrm{MJ}</math>
| |
| | |
| The amount of various molecules in an oil sample can be determined in laboratory. The molecules are typically extracted in a [[solvent]], then separated in a [[gas chromatograph]], and finally determined with a suitable [[detector]], such as a [[flame ionization detector]] or a [[mass spectrometer]]<ref>Use of ozone depleting substances in laboratories. TemaNord 2003:516. http://www.norden.org/pub/ebook/2003-516.pdf</ref>.
| |
| | |
| Incomplete combustion of petroleum or gasoline results in production of toxic byproducts. Too little oxygen results in [[carbon monoxide]]. Due to the high temperatures and high pressures involved, exhaust gases from gasoline combustion in car engines usually include [[nitrogen oxide]]s which are responsible for creation of [[photochemical smog]].
| |
| | |
| ==Formation==
| |
| According to generally accepted theory, petroleum is derived from ancient [[biomass]].<ref>Keith A. Kvenvolden “Organic geochemistry – A retrospective of its first 70 years” Organic Geochemistry 37 (2006) 1–11. {{DOI|10.1016/j.orggeochem.2005.09.001}}</ref> The theory was initially based on the isolation of molecules from petroleum that closely resemble known biomolecules (Figure).
| |
| [[File:Treibs&Chlorophyll.png|thumb|350 px| center|Structure of vanadium [[porphyrin]] compound extracted from petroleum by Alfred Treibs, father of [[organic geochemistry]]. Treibs noted the close structural similarity of this molecule and [[chlorophyll]] a.]]
| |
| | |
| More specifically, crude oil and [[natural gas]] are products of [[diagenesis|heating]] of ancient [[organic compound|organic materials]] (i.e. [[kerogen]]) over [[geologic time scale|geological time]]. Formation of petroleum occurs from [[hydrocarbon]] [[pyrolysis]], in a variety of mostly [[endothermic]] reactions at high temperature and/or pressure.<ref>[http://www.osti.gov/bridge/servlets/purl/10169154-cT5xip/10169154.PDF Petroleum Study]</ref> Today's oil formed from the preserved remains of [[prehistory|prehistoric]] [[zooplankton]] and [[algae]], which had settled to a sea or lake bottom in large quantities under [[anoxic sea water|anoxic conditions]] (the remains of prehistoric [[terrestrial plant]]s, on the other hand, tended to form [[coal]]). Over geological time the organic matter mixed with [[mud]], and was buried under heavy layers of [[sediment]] resulting in high levels of [[heat]] and [[pressure]] (known as diagenesis). This process caused the organic matter to change, first into a waxy material known as kerogen, which is found in various [[oil shale]]s around the world, and then with more heat into liquid and gaseous hydrocarbons via a process known as [[catagenesis (geology)|catagenesis]].
| |
| | |
| Geologists often refer to the temperature range in which oil forms as an "oil window"—below the minimum temperature oil remains trapped in the form of kerogen, and above the maximum temperature the oil is converted to [[natural gas]] through the process of [[thermal cracking]]. Although this temperature range is found at different depths below the surface throughout the world, a typical depth for the oil window is 4–6 km. Sometimes, oil which is formed at extreme depths may migrate and become trapped at much shallower depths than where it was formed. The [[Athabasca Oil Sands]] is one example of this.
| |
| | |
| ===Abiogenic origin===
| |
| {{main|Abiogenic petroleum origin}}
| |
| A number of geologists in Russia adhere to the [[abiogenic petroleum origin]] hypothesis and maintain that hydrocarbons of purely inorganic origin exist within Earth's interior. Astronomer [[Thomas Gold]] championed the theory in the [[Western world]] by supporting the work done by [[Nikolai Kudryavtsev]] in the 1950s. It is currently supported primarily by Kenney and Krayushkin.<ref>[http://www.gasresources.net/DisposalBioClaims.htm Kenney et al., Dismissal of the Claims of a Biological Connection for Natural Petroleum, Energia 2001]</ref>
| |
| | |
| The abiogenic origin hypothesis lacks scientific support. Extensive research into the chemical structure of kerogen has identified algae as the primary source of oil. The abiogenic origin hypothesis fails to explain the presence of these markers in kerogen and oil, as well as failing to explain how inorganic origin could be achieved at temperatures and pressures sufficient to convert kerogen to graphite. It has not been successfully used in uncovering oil deposits by geologists, as the hypothesis lacks any mechanism for determining where the process may occur.<ref> {{cite journal
| |
| |last= Glasby
| |
| |first=Geoffrey P.
| |
| |date=2006
| |
| |title= Abiogenic origin of hydrocarbons: an historical overview
| |
| |journal= Resource Geology
| |
| |volume=56
| |
| |issue=1
| |
| |pages=83–96
| |
| |url=http://static.scribd.com/docs/j79lhbgbjbqrb.pdf
| |
| |format=PDF
| |
| |accessdate=2008-02-17
| |
| |doi= 10.1111/j.1751-3928.2006.tb00271.x}}
| |
| </ref>
| |
| More recently scientists at the Carnegie Institution for Science have found that ethane and heavier hydrocarbons can be synthesized under conditions of the upper mantle.<ref>[http://www.eurekalert.org/pub_releases/2009-07/ci-hit072409.php Hydrocarbons in the deep Earth?] July 2009 news release.</ref>
| |
| | |
| == Crude oil ==
| |
| ===Crude oil reservoirs===
| |
| [[Image:Structural Trap (Anticlinal).svg|thumb|140px|Hydrocarbon trap.]]
| |
| Three conditions must be present for oil reservoirs to form: a [[source rock]] rich in hydrocarbon material buried deep enough for subterranean heat to cook it into oil; a [[porous]] and [[permeability (fluid)|permeable]] reservoir rock for it to accumulate in; and a cap rock (seal) or other mechanism that prevents it from escaping to the surface. Within these reservoirs, fluids will typically organize themselves like a three-layer cake with a layer of water below the oil layer and a layer of gas above it, although the different layers vary in size between reservoirs.
| |
| Because most hydrocarbons are [[buoyancy|lighter]] than rock or water, they often migrate upward through adjacent rock layers until either reaching the surface or becoming trapped within porous rocks (known as [[oil reservoir|reservoirs]]) by impermeable rocks above. However, the process is influenced by underground water flows, causing oil to migrate hundreds of kilometres horizontally or even short distances downward before becoming trapped in a reservoir. When hydrocarbons are concentrated in a trap, an [[oil field]] forms, from which the liquid can be extracted by [[drill]]ing and [[pump]]ing.
| |
| | |
| The reactions that produce oil and natural gas are often modeled as first order breakdown reactions, where hydrocarbons are broken down to oil and natural gas by a set of parallel reactions, and oil eventually breaks down to natural gas by another set of reactions. The latter set is regularly used in [[petrochemical]] plants and [[oil refineries]].
| |
| | |
| ===Unconventional oil reservoirs===
| |
| {{see also|Unconventional oil|Oil sands|Oil shale reserves}}
| |
| Oil-eating bacteria [[biodegradation|biodegrades]] oil that has escaped to the surface. [[Oil sands]] are reservoirs of partially biodegraded oil still in the process of escaping and being biodegraded, but they contain so much migrating oil that, although most of it has escaped, vast amounts are still present—more than can be found in conventional oil reservoirs. The lighter fractions of the crude oil are destroyed first, resulting in reservoirs containing an extremely heavy form of crude oil, called crude bitumen in Canada, or extra-heavy crude oil in Venezuela. These two countries have the world's largest deposits of oil sands.
| |
| | |
| On the other hand, [[oil shale]]s are source rocks that have not been exposed to heat or pressure long enough to convert their trapped hydrocarbons into crude oil. Technically speaking, oil shales are not really shales and do not really contain oil, but are usually relatively hard rocks called [[marl]]s containing a waxy substance called [[kerogen]]. The kerogen trapped in the rock can be converted into crude oil using heat and pressure to simulate natural processes. The method has been known for centuries and was patented in 1694 under British Crown Patent No. 330 covering, "A way to extract and make great quantityes of pitch, tarr, and oyle out of a sort of stone." Although oil shales are found in many countries, the United States has the world's largest deposits.<ref name=Lambertson>{{cite news | title=Oil Shale: Ready to Unlock the Rock | first=Giles | last=Lambertson | publisher=Construction Equipment Guide | url=http://www.cegltd.com/story.asp?story=10092 | date=2008-02-16 | accessdate=2008-05-21}}</ref>
| |
| | |
| ==Classification==
| |
| {{see also|Benchmark (crude oil)}}
| |
| [[Image:Petroleum.JPG|thumb|right|250px|A sample of medium heavy crude oil]]
| |
| | |
| The [[petroleum industry]] generally classifies crude oil by the geographic location it is produced in (e.g. [[West Texas Intermediate]], [[Brent oilfield|Brent]], or [[Oman]]), its [[API gravity]] (an oil industry measure of density), and by its sulfur content. Crude oil may be considered ''[[Light crude oil|light]]'' if it has low density or ''[[Heavy crude oil|heavy]]'' if it has high density; and it may be referred to as [[sweet crude oil|sweet]] if it contains relatively little sulfur or ''[[sour crude oil|sour]]'' if it contains substantial amounts of sulfur.
| |
| | |
| The geographic location is important because it affects transportation costs to the refinery. ''Light'' crude oil is more desirable than ''heavy'' oil since it produces a higher yield of gasoline, while ''sweet'' oil commands a higher price than ''sour'' oil because it has fewer environmental problems and requires less refining to meet sulfur standards imposed on fuels in consuming countries. Each crude oil has unique molecular characteristics which are understood by the use of [[Crude oil assay|crude oil assay analysis]] in petroleum laboratories.
| |
| | |
| [[barrel (unit)|Barrel]]s from an area in which the crude oil's molecular characteristics have been determined and the oil has been classified are used as pricing [[Benchmark (crude oil)|references]] throughout the world. Some of the common reference crudes are:
| |
| | |
| * [[West Texas Intermediate]] (WTI), a very high-quality, sweet, light oil delivered at [[Cushing, Oklahoma]] for North American oil
| |
| * [[Brent Crude|Brent Blend]], comprising 15 oils from fields in the [[Brent oilfield|Brent]] and [[Ninian]] systems in the [[East Shetland Basin]] of the [[North Sea]]. The oil is landed at [[Sullom Voe]] terminal in the [[Shetlands]]. Oil production from Europe, Africa and Middle Eastern oil flowing West tends to be priced off this oil, which forms a [[Benchmark (crude oil)|benchmark]]
| |
| * [[Dubai Crude|Dubai-Oman]], used as benchmark for Middle East sour crude oil flowing to the [[Asia]]-[[Pacific]] region
| |
| * [[Tapis]] (from [[Malaysia]], used as a reference for light Far East oil)
| |
| * Minas (from [[Indonesia]], used as a reference for heavy Far East oil)
| |
| * The [[OPEC Reference Basket]], a weighted average of oil blends from various [[OPEC]] (The Organization of the Petroleum Exporting Countries) countries
| |
| | |
| There are declining amounts of these benchmark oils being produced each year, so other oils are more commonly what is actually delivered. While the reference price may be for West Texas Intermediate delivered at Cushing, the actual oil being traded may be a discounted Canadian heavy oil delivered at [[Hardisty, Alberta]], and for a Brent Blend delivered at the Shetlands, it may be a Russian Export Blend delivered at the port of [[Primorsk, Leningrad Oblast|Primorsk]].<ref>{{cite web
| |
| | title = Light Sweet Crude Oil
| |
| | work = About the Exchange
| |
| | publisher = New York Mercantile Exchange (NYMEX)
| |
| | date = 2006
| |
| | url = http://www.nymex.com/lsco_fut_descri.aspx
| |
| | accessdate = 2008-04-21}}
| |
| </ref>
| |
| | |
| ==Petroleum industry==
| |
| [[Image:WTI price 96 09.svg|thumbnail|right|250px|[[New York Mercantile Exchange]] prices for West Texas Intermediate 1996–2009]]
| |
| {{main|Petroleum industry}}
| |
| | |
| The petroleum industry is involved in the global processes of [[Hydrocarbon exploration|exploration]], [[Extraction of petroleum|extraction]], [[Oil refinery|refining]], [[Petroleum transport|transporting]] (often with [[oil tanker]]s and [[Pipeline transport|pipelines]]), and marketing petroleum products. The largest volume products of the industry are [[fuel oil]] and [[gasoline]] (petrol). Petroleum is also the raw material for many [[Petrochemical|chemical products]], including pharmaceuticals, solvents, fertilizers, pesticides, and plastics. The industry is usually divided into three major components: [[Upstream (oil industry)|upstream]], [[midstream]] and [[Downstream (oil industry)|downstream]]. Midstream operations are usually included in the downstream category.
| |
| | |
| Petroleum is vital to many [[industries]], and is of importance to the maintenance of industrialized [[civilization]] itself, and thus is critical concern to many nations. Oil accounts for a large percentage of the world's energy consumption, ranging from a low of 32% for [[Europe]] and Asia, up to a high of 53% for the [[Middle East]]. Other geographic regions' consumption patterns are as follows: South and [[Central America]] (44%), [[Africa]] (41%), and [[North America]] (40%). The world at large consumes 30 billion [[Barrel (unit)|barrels]] (4.8 km³) of oil per year, and the top oil consumers largely consist of developed nations. In fact, 24% of the oil consumed in 2004 went to the [[United States]] alone <ref> {{cite web
| |
| |title=International Energy Annual 2004
| |
| |publisher=Energy Information Administration
| |
| |date=2006-07-14
| |
| |url=http://www.eia.doe.gov/pub/international/iealf/tablee2.xls
| |
| |format=XLS}}<!-- For annual updates use the search at http://www.eia.doe.gov/ for terms "International Energy Annual 2006" "Tables" "Petroleum consumption" --></ref>, though by 2007 this had dropped to 21% of world oil consumed.<ref>{{cite web
| |
| |title=Yearbook 2008 - crude oil
| |
| |publisher=Enerdata
| |
| |url=http://www.enerdata.fr/enerdatauk/publications/yearbook/crude_oil.php}}
| |
| </ref>
| |
|
| |
| In the US, in the states of [[Arizona]], [[California]], [[Hawaii]], [[Nevada]], [[Oregon]] and [[Washington]], the Western States Petroleum Association (WSPA) is responsible for producing, distributing, refining, transporting and marketing petroleum. This non-profit trade association was founded in 1907, and is the oldest petroleum trade association in the United States.<ref>
| |
| {{cite web
| |
| |url=http://www.wspa.org/about/index.htm
| |
| |title=Western States Petroleum Association - About Us
| |
| |accessdate=2008-11-03
| |
| }}</ref>
| |
| | |
| ==History==
| |
| {{main|History of petroleum}}
| |
| [[Image:Gusher Okemah OK 1922.jpg|thumb|Oil derrick in [[Okemah, Oklahoma]], 1922.]]
| |
| Petroleum, in one form or another, has been used since ancient times, and is now important across society, including in economy, politics and technology. The rise in importance was mostly due to the invention of the [[internal combustion engine]].
| |
| | |
| More than 4000 years ago, according to [[Herodotus]] and [[Diodorus Siculus]], [[asphalt]] was used in the construction of the walls and towers of [[Babylon]]; there were oil pits near [[Ardericca]] (near Babylon), and a pitch spring on [[Zacynthus]].<ref name=EB1911>{{1911|article=Petroleum}}</ref> Great quantities of it were found on the banks of the river [[Issus (river)|Issus]], one of the tributaries of the [[Euphrates]]. Ancient [[Persian Empire|Persian]] tablets indicate the medicinal and lighting uses of petroleum in the upper levels of their society.
| |
| | |
| Today, about 90% of vehicular fuel needs are met by oil. Petroleum also makes up 40% of total energy consumption in the [[United States]], but is responsible for only 2% of electricity generation. Petroleum's worth as a portable, dense energy source powering the vast majority of vehicles and as the base of many industrial chemicals makes it one of the world's most important [[commodity|commodities]].
| |
| | |
| The top three oil producing countries are [[Saudi Arabia]], [[Russia]], and the [[United States]].<ref>[http://www.infoplease.com/ipa/A0922041.html InfoPlease]</ref> About 80% of the world's readily accessible reserves are located in the [[Middle East]], with 62.5% coming from the Arab 5: [[Saudi Arabia]], [[UAE]], [[Iraq]], [[Qatar]] and [[Kuwait]]. A large portion of the world's total oil exists as unconventional sources, such as [[bitumen]] in [[Athabasca oil sands|Canada]] and [[Orinoco Belt|Venezuela]] and [[oil shale]]. While significant volumes of oil are extracted from oil sands, particularly in Canada, logistical and technical hurdles remain, and Canada's oil sands are not expected to provide more than a few million barrels per day in the foreseeable future.
| |
| | |
| == Price ==
| |
| {{Main|Price of petroleum}}
| |
| After the collapse of the OPEC-administered pricing system in 1985, and a short lived experiment with netback pricing, oil-exporting countries adopted a market-linked pricing mechanism.<ref name="Mabro">{{cite book|last=Mabro|first=Robert|coauthors=Organization of Petroleum Exporting Countries|title=Oil in the 21st century: issues, challenges and opportunities|publisher=Oxford Press|date=2006|pages=351|isbn=0199207380, 9780199207381}}</ref> First adopted by [[PEMEX]] in 1986, market-linked pricing was widely accepted, and by 1988 became and still is the main method for pricing crude oil in international trade.<ref name="Mabro" /> The current reference, or pricing markers, are [[Brent Crude|Brent]], [[West Texas Intermediate|WTI]] , and [[Dubai Crude|Dubai/Oman]].<ref name="Mabro" />
| |
| | |
| ==Uses==
| |
| The chemical structure of petroleum is [[Heterogeneity|heterogeneous]] (composed of [[hydrocarbon]] chains of different lengths). Because of this, petroleum may be taken to [[oil refinery|oil refineries]] and the hydrocarbon chemicals separated by [[distillation]] and treated by other [[chemical process]]es, to be used for a variety of purposes. See [[Petroleum product]]s.
| |
| [[Image:Santamonicafreewaynearrobertson.jpg|thumb|right|230px|A traffic jam on a typical American freeway, the [[Santa Monica Freeway]] in [[Los Angeles]].]]
| |
| ===Fuels===
| |
| The most common [[Petroleum distillation|distillation]]s of petroleum are [[fuel]]s.
| |
| Fuels include:
| |
| | |
| *[[Ethane]] and other short-chain [[alkanes]]
| |
| *[[Diesel fuel]] (petrodiesel)
| |
| *[[Fuel oil]]s
| |
| *[[Gasoline]] (Petrol)
| |
| *[[Jet fuel]]
| |
| *[[Kerosene]]
| |
| *[[Liquefied petroleum gas]] (LPG)
| |
| | |
| ===Other derivatives===
| |
| Certain types of resultant hydrocarbons may be mixed with other non-hydrocarbons, to create other end products:
| |
| | |
| *[[Alkenes]] (olefins) which can be manufactured into [[plastics]] or other compounds
| |
| *[[Lubricant]]s (produces light machine oils, [[motor oil]]s, and [[Grease (lubricant)|grease]]s, adding [[viscosity]] stabilizers as required).
| |
| *[[Wax]], used in the packaging of [[frozen food]]s, among others.
| |
| *[[Sulfur]] or [[Sulfuric acid]]. These are a useful industrial materials. Sulfuric acid is usually prepared as the acid precursor [[oleum]], a byproduct of [[Hydrodesulfurization|sulfur removal]] from fuels.
| |
| *Bulk [[tar]].
| |
| *[[Asphalt]]
| |
| *[[Petroleum coke]], used in speciality carbon products or as solid fuel.
| |
| *[[Paraffin wax]]
| |
| *[[Aromatic]] [[petrochemical]]s to be used as precursors in other [[chemical]] production.
| |
| | |
| ==Petroleum by country==
| |
| <!-- this was a left over from a previous incarnation of the section. It is no longer a useful link. I looked around for a better link but didn't find one. Feel free to create an article on consumption/production etc. by country {{main|Petroleum Industry}} -->
| |
| | |
| ===Consumption statistics===
| |
| <gallery>
| |
| Image:Global Carbon Emission by Type to Y2004.png|Global fossil carbon emissions, an indicator of consumption, for 1800 - 2004. Total is black, Oil is in blue.
| |
| Image:EIA_IEO2006.jpg|World energy consumption, 1980 - 2030. ''Source: International Energy Outlook 2006.''
| |
| Image:Oil consumption per day by region from 1980 to 2006.svg|daily oil consumption from 1980 to 2006
| |
| Image:Oil consumption per day by region from 1980 to 2006 solid3.svg|oil consumption by percentage of total per region from 1980 to 2006: <font style="color:red">'''red'''</font>=USA, <font style="color:blue">'''blue'''</font>=Europe, <font style="color:#D1D117">'''yellow'''</font>=Asia+Oceania
| |
| </gallery>
| |
| | |
| ===Consumption===
| |
| <!-- Image with unknown copyright status removed: [[Image:WorldPetroleum2007.png|thumb|center|550px|Mean oil production by country in 2007, shown as a percentage of the top producer (Saudi Arabia - 10.2 millions of barrels per day).]] -->
| |
| [[Image:OilConsumptionpercapita.png|thumb|center|550px|Oil consumption per capita (darker colors represent more consumption).]]
| |
| | |
| This table orders the amount of petroleum consumed in 2006 in thousand [[Barrel (unit)|barrels]] (bbl) per day and in thousand [[cubic metre]]s (m<sup>3</sup>) per day:<ref>U.S. Energy Information Administration. Excel file RecentPetroleumConsumptionBarrelsperDay.xls from web page http://tonto.eia.doe.gov/dnav/pet/pet_pri_wco_k_w.htm (direct link: http://www.eia.doe.gov/emeu/international/RecentPetroleumConsumptionBarrelsperDay.xls) "Table Posted: November 7, 2008"</ref><ref>From DSW-Datareport 2006 ("Deutsche Stiftung Weltbevölkerung")</ref><ref>One cubic metre of oil is equivalent to 6.28981077 barrels of oil</ref>
| |
| {| style="text-align: right;" border="1" cellspacing="0" class="wikitable sortable"
| |
| !Consuming Nation 2006
| |
| !(1000 bbl/day)
| |
| !(1000 m<sup>3</sup>/day)
| |
| !population in millions
| |
| !bbl/year per capita
| |
| |-
| |
| |{{rh}}|[[United States]] <sup>1</sup> || {{convert|20687.42|oilbbl|m3|1|disp=table}} || 304
| |
| |{{round|{{#expr:365*20687.42/304000}}|1}}
| |
| |-
| |
| |{{rh}}|[[China]] || {{convert|7201.28|oilbbl|m3|1|disp=table}} || 1369
| |
| |{{round|{{#expr:365*7274/1369000}}|1}}
| |
| |- | | |- |
| |{{rh}}|[[Japan]] <sup>2</sup> || {{convert|5197.70|oilbbl|m3|1|disp=table}} || 128 | | |align=right|where: |
| |{{round|{{#expr:365*5197.7/128000}}|1}} | | | |
| |- | | |- |
| |{{rh}}|[[Russia]] <sup>1</sup> || {{convert|2810.76|oilbbl|m3|1|disp=table}} || 142 | | !align=right|<math>f</math> |
| |{{round|{{#expr:365*2810.76/142000}}|1}}
| | |align=left|= crosswind dispersion parameter |
| |- | | |- |
| |{{rh}}|[[Germany]] <sup>2</sup> || {{convert|2691.81|oilbbl|m3|1|disp=table}} || 82 | | !align=right| |
| |{{round|{{#expr:365*2691.81/82000}}|1}}
| | |align=left|= <math>\exp\;[-\,y^2/\,(2\;\sigma_y^2\;)\;]</math> |
| |- | | |- |
| |{{rh}}|[[India]] <sup>2</sup> || {{convert|2571.90|oilbbl|m3|1|disp=table}} || 1201 | | !align=right|<math>g</math> |
| |{{round|{{#expr:365*2571.90/1201000}}|1}}
| | |align=left|= vertical dispersion parameter = <math>\,g_1 + g_2 + g_3</math> |
| |- | | |- |
| |{{rh}}|[[Canada]] || {{convert|2296.66|oilbbl|1|m3|1|disp=table}} || 32<ref>{{cite web |author=Beauchesne, Eric |publisher=National Post |url=http://www.canada.com/nationalpost/financialpost/story.html?id=73b94aac-08f0-477f-a72a-b8b640f6658f&k=90795 |title=We are 31,612,897 |date=2007-03-13 |accessdate=2008-11-11}}</ref>
| | !align=right|<math>g_1</math> |
| |{{round|{{#expr:365*2296.66/31613}}|1}} | | |align=left|= vertical dispersion with no reflections |
| |- | | |- |
| |{{rh}}|[[Brazil]] || {{convert|2216.84|oilbbl|1|m3|1|disp=table}} || 187
| | !align=right| |
| |{{round|{{#expr:365*2216.84/187000}}|1}} | | |align=left|= <math>\; \exp\;[-\,(z - H)^2/\,(2\;\sigma_z^2\;)\;]</math> |
| |- | | |- |
| |{{rh}}|[[South Korea]] <sup>2</sup> || {{convert|2179.90|oilbbl|1|m3|1|disp=table}} || 49<ref>IndexMundi. [http://indexmundi.com/south_korea/population.html South Korea Population - Demographics]. "48,846,823" ... "July 2006 est." Retrieved 2008-11-11</ref><!-- CIA estimates 48,379,392 for July 2008, http://www.korea.net/korea/kor_loca.asp?code=L03 for end of 2007 has 48,456,369 --> | | !align=right|<math>g_2</math> |
| |{{round|{{#expr:365*2179.9041/48847}}|1}}
| | |align=left|= vertical dispersion for reflection from the ground |
| |- | | |- |
| |{{rh}}|[[Saudi Arabia]] ([[OPEC]]) || {{convert|2139.42|oilbbl|1|m3|1|disp=table}} || 27<ref>Sources vary: 24,600,000 from {{cite web |title=UNHCR / Refworld / The Worst of the Worst 2006 - Saudi Arabia |url=http://www.unhcr.org/refworld/docid/4917f8351e.html |publisher=[[United Nations High Commissioner for Refugees]] |accessdate=2008-11-11}}; while IndexMundi listed a July 2006 estimate of 27,019,73: {{cite web |title=Saudi Arabia Population - Demographics |url=http://indexmundi.com/saudi_arabia/population.html |publisher=IndexMundi |accessdate=2008-11-11}}</ref> | | !align=right| |
| |{{round|{{#expr:365*2139.42/27000}}|1}}
| | |align=left|= <math>\;\exp\;[-\,(z + H)^2/\,(2\;\sigma_z^2\;)\;]</math> |
| |- | | |- |
| |{{rh}}|[[Mexico]] <sup>1</sup> || {{convert|2077.51|oilbbl|1|m3|1|disp=table}} || 107 | | !align=right|<math>g_3</math> |
| |{{round|{{#expr:365*2077.51/107000}}|1}}
| | |align=left|= vertical dispersion for reflection from an inversion aloft |
| |- | | |- |
| |{{rh}}|[[France]] <sup>2</sup>|| {{convert|1981.18|oilbbl|1|m3|1|disp=table}} || 61<ref>IndexMundi. [http://indexmundi.com/france/population.html France Population - Demographics]. "60,876,136" ... "July 2006 est." Retrieved 2008-11-11</ref> | | !align=right| |
| |{{round|{{#expr:365*1981.1781/60876}}|1}}
| | |align=left|= <math>\sum_{m=1}^\infty\;\big\{\exp\;[-\,(z - H - 2mL)^2/\,(2\;\sigma_z^2\;)\;]</math> |
| |- | | |- |
| |{{rh}}|[[United Kingdom]] <sup>1</sup> || {{convert|1812.01|oilbbl|1|m3|1|disp=table}} || 61<ref>IndexMundi. [http://indexmundi.com/united_kingdom/population.html United Kingdom Population - Demographics]. "60,609,153" ... "July 2006 est." Retrieved 2008-11-11</ref> | | !align=right| |
| |{{round|{{#expr:365*1812.0055/60609}}|1}}
| | |align=left| <math>+\, \exp\;[-\,(z + H + 2mL)^2/\,(2\;\sigma_z^2\;)\;]</math> |
| |- | | |- |
| |{{rh}}|[[Italy]] <sup>2</sup>|| {{convert|1742.58|oilbbl|1|m3|1|disp=table}} || 58<ref>IndexMundi. [http://indexmundi.com/italy/population.html Italy Population - Demographics]. "58,133,509" ... "July 2006 est." Retrieved 2008-11-11</ref> | | !align=right| |
| |{{round|{{#expr:365*1742.5836/58134}}|1}}
| | |align=left| <math>+\, \exp\;[-\,(z + H - 2mL)^2/\,(2\;\sigma_z^2\;)\;]</math> |
| |- | | |- |
| |{{rh}}|[[Iran]] ([[OPEC]])|| {{convert|1679.20|oilbbl|1|m3|1|disp=table}} || 68<ref>IndexMundi. [http://indexmundi.com/iran/population.html Iran Population - Demographics]. "68,688,433" ... "July 2006 est." Retrieved 2008-11-11</ref> | | !align=right| |
| |{{round|{{#expr:365*1679.2005852/68688}}|1}}
| | |align=left| <math>+\, \exp\;[-\,(z - H + 2mL)^2/\,(2\;\sigma_z^2\;)\;]\big\}</math> |
| |- | | |- |
| |} | | !align=right|<math>C</math> |
| Source: [http://www.eia.doe.gov/emeu/cabs/topworldtables1_2.htm US Energy Information Administration]
| | |align=left|= concentration of emissions, in g/m³, at any receptor located: |
| | |
| <small><sup>1</sup> [[Oil reserves#Countries that have already passed their production peak|peak production of oil already passed in this state]]</small>
| |
| | |
| <small><sup>2</sup> This country is not a major oil producer</small>
| |
| | |
| ===Production===
| |
| | |
| [[Image:Oil producing countries map.png|thumb|center|450px|Oil producing [[List of oil-producing states|countries]]]]
| |
| | |
| [[Image:Top Oil Producing Counties.png|thumb|Graph of Top Oil Producing Countries 1960-2006, including Soviet Union<ref>http://www.eia.doe.gov/emeu/aer/pdf/pages/sec11_10.pdf</ref>]]
| |
| | |
| In petroleum industry parlance, ''production'' refers to the quantity of crude extracted from reserves, not the literal creation of the product.
| |
| | |
| {| style="text-align: right;" border="1" cellspacing="0" class="wikitable sortable"
| |
| !#
| |
| !Producing Nation
| |
| !10<sup>3</sup>bbl/d (2006)
| |
| !10<sup>3</sup>bbl/d (2007)
| |
| |- | | |- |
| |1 | | !align=right| |
| |{{rh}}|[[Saudi Arabia]] ([[OPEC]]) | | |align=left| x meters downwind from the emission source point |
| |10,665
| |
| |10,234
| |
| |- | | |- |
| |2 | | !align=right| |
| |{{rh}}|[[Russia]] <sup>1</sup> | | |align=left| y meters crosswind from the emission plume centerline |
| |9,677
| |
| |9,876
| |
| |- | | |- |
| |3 | | !align=right| |
| |{{rh}}|[[United States]] <sup>1</sup> | | |align=left| z meters above ground level |
| |8,331
| |
| |8,481
| |
| |- | | |- |
| |4 | | !align=right|<math>Q</math> |
| |{{rh}}|[[Iran]] (OPEC) | | |align=left|= source pollutant emission rate, in g/s |
| |4,148
| |
| |4,043
| |
| |- | | |- |
| |5 | | !align=right|<math>u</math> |
| |{{rh}}|[[China]] | | |align=left|= horizontal wind velocity along the plume centerline, m/s |
| |3,845
| |
| |3,901
| |
| |- | | |- |
| |6 | | !align=right|<math>H</math> |
| |{{rh}}|[[Mexico]] <sup>1</sup>
| | |align=left|= height of emission plume centerline above ground level, in m |
| |3,707 | |
| |3,501 | |
| |- | | |- |
| |7 | | !align=right|<math>\sigma_z</math> |
| |{{rh}}|[[Canada]] <sup>2</sup>
| | |align=left|= vertical standard deviation of the emission distribution, in m |
| |3,288 | |
| |3,358 | |
| |- | | |- |
| |8 | | !align=right|<math>\sigma_y</math> |
| |{{rh}}|[[United Arab Emirates]] (OPEC) | | |align=left|= horizontal standard deviation of the emission distribution, in m |
| |2,945
| |
| |2,948
| |
| |- | | |- |
| |9 | | !align=right|<math>L</math> |
| |{{rh}}|[[Venezuela]] (OPEC) <sup>1</sup>
| | |align=left|= height from ground level to bottom of the inversion aloft, in m |
| |2,803 | |
| |2,667 | |
| |- | | |- |
| |10 | | !align=right|<math>\exp</math> |
| |{{rh}}|[[Kuwait]] (OPEC)
| | |align=left|= the exponential function |
| |2,675
| |
| |2,613
| |
| |-
| |
| |11
| |
| |{{rh}}|[[Norway]] <sup>1</sup>
| |
| |2,786 | |
| |2,565
| |
| |-
| |
| |12
| |
| |{{rh}}|[[Nigeria]] (OPEC)
| |
| |2,443
| |
| |2,352
| |
| |-
| |
| |13
| |
| |{{rh}}|[[Brazil]]
| |
| |2,166
| |
| |2,279
| |
| |-
| |
| |14
| |
| |{{rh}}|[[Algeria]] (OPEC)
| |
| |2,122
| |
| |2,173
| |
| |-
| |
| |15
| |
| |{{rh}}|[[Iraq]] (OPEC) <sup>3</sup>
| |
| |2,008
| |
| |2,094
| |
| |-
| |
| |16
| |
| |{{rh}}|[[Libya]] (OPEC)
| |
| |1,809
| |
| |1,845
| |
| |-
| |
| |17
| |
| |{{rh}}|[[Angola]] (OPEC)
| |
| |1,435
| |
| |1,769
| |
| |-
| |
| |18
| |
| |{{rh}}|[[United Kingdom]]
| |
| |1,689
| |
| |1,690
| |
| |-
| |
| |19
| |
| |{{rh}}|[[Kazakhstan]]
| |
| |1,388
| |
| |1,445
| |
| |-
| |
| |20
| |
| |{{rh}}|[[Qatar]] (OPEC)
| |
| |1,141
| |
| |1,136
| |
| |-
| |
| |21
| |
| |{{rh}}|[[Indonesia]]
| |
| |1,102
| |
| |1,044
| |
| |-
| |
| |22
| |
| |{{rh}}|[[India]]
| |
| |854
| |
| |881
| |
| |-
| |
| |23
| |
| |{{rh}}|[[Azerbaijan]]
| |
| |648
| |
| |850
| |
| |-
| |
| |24
| |
| |{{rh}}|[[Argentina]]
| |
| |802
| |
| |791
| |
| |-
| |
| |25
| |
| |{{rh}}|[[Oman]]
| |
| |743
| |
| |714
| |
| |-
| |
| |26
| |
| |{{rh}}|[[Malaysia]]
| |
| |729
| |
| |703
| |
| |-
| |
| |27
| |
| |{{rh}}|[[Egypt]]
| |
| |667
| |
| |664
| |
| |-
| |
| |28
| |
| |{{rh}}|[[Australia]]
| |
| |552
| |
| |595
| |
| |-
| |
| |29
| |
| |{{rh}}|[[Colombia]]
| |
| |544
| |
| |543
| |
| |-
| |
| |30
| |
| |{{rh}}|[[Ecuador]] (OPEC)
| |
| |536
| |
| |512
| |
| |-
| |
| |31
| |
| |{{rh}}|[[Sudan]]
| |
| |380
| |
| |466
| |
| |-
| |
| |32
| |
| |{{rh}}|[[Syria]]
| |
| |449
| |
| |446
| |
| |-
| |
| |33
| |
| |{{rh}}|[[Equatorial Guinea]]
| |
| |386
| |
| |400
| |
| |-
| |
| |34
| |
| |{{rh}}|[[Yemen]]
| |
| |377 | |
| |361
| |
| |-
| |
| |35
| |
| |{{rh}}|[[Vietnam]]
| |
| |362
| |
| |352
| |
| |-
| |
| |36
| |
| |{{rh}}|[[Thailand]]
| |
| |334
| |
| |349
| |
| |-
| |
| |37
| |
| |{{rh}}|[[Denmark]]
| |
| |344
| |
| |314
| |
| |-
| |
| |38
| |
| |{{rh}}|[[Republic of the Congo|Congo]]
| |
| |247
| |
| |250
| |
| |-
| |
| |39
| |
| |{{rh}}|[[Gabon]]
| |
| |237
| |
| |244
| |
| |-
| |
| |40
| |
| |{{rh}}|[[South Africa]]
| |
| |204
| |
| |199
| |
| |} | | |} |
| Source: [http://tonto.eia.doe.gov/country/index.cfm U.S. Energy Information Administration]
| |
|
| |
|
| <small><sup>1</sup> Peak production of conventional oil already passed in this state.
| | The above equation not only includes upward reflection from the ground, it also includes downward reflection from the bottom of any inversion lid present in the atmosphere. |
|
| |
|
| <sup>2</sup> Although Canadian conventional oil production is declining, total oil production is increasing as oil sands production grows. If oil sands are included, it has the world's second largest oil reserves after Saudi Arabia. | | The sum of the four exponential terms in <math>g_3</math> converges to a final value quite rapidly. For most cases, the summation of the series with '''''m''''' = 1, '''''m''''' = 2 and '''''m''''' = 3 will provide an adequate solution. |
| | |
| <sup>3</sup> Though still a member, Iraq has not been included in production figures since 1998</small>
| |
| | |
| ===Export=== | |
| See also: [[Fossil fuel exporters]]
| |
| [[Image:Oil exports.PNG|thumb|300px|Oil exports by country]]
| |
| | |
| In order of net exports in 2006 in thousand [[Barrel (unit)|bbl]]/[[Day|d]] and thousand [[Cubic metre|m³]]/d:
| |
| {| style="text-align: right;" border="1" cellspacing="0" class="wikitable sortable"
| |
| !#
| |
| !Exporting Nation (2006)
| |
| !(10<sup>3</sup>bbl/d)
| |
| !(10<sup>3</sup>m<sup>3</sup>/d)
| |
| |-
| |
| |1
| |
| |{{rh}}|[[Saudi Arabia]] ([[OPEC]])
| |
| |8,651
| |
| |1,376
| |
| |-
| |
| |2
| |
| |{{rh}}|[[Russia]] <sup>1</sup>
| |
| |6,565
| |
| |1,044
| |
| |-
| |
| |3
| |
| |{{rh}}|[[Norway]] <sup>1</sup>
| |
| |2,542
| |
| |404
| |
| |-
| |
| |4
| |
| |{{rh}}|[[Iran]] (OPEC)
| |
| |2,519
| |
| |401
| |
| |-
| |
| |5
| |
| |{{rh}}|[[United Arab Emirates]] (OPEC)
| |
| |2,515
| |
| |400
| |
| |-
| |
| |6
| |
| |{{rh}}|[[Venezuela]] (OPEC) <sup>1</sup>
| |
| |2,203
| |
| |350
| |
| |-
| |
| |7
| |
| |{{rh}}|[[Kuwait]] (OPEC)
| |
| |2,150
| |
| |342
| |
| |-
| |
| |8
| |
| |{{rh}}|[[Nigeria]] (OPEC)
| |
| |2,146
| |
| |341
| |
| |-
| |
| |9
| |
| |{{rh}}|[[Algeria]] (OPEC) <sup>1</sup>
| |
| |1,847
| |
| |297
| |
| |-
| |
| |10
| |
| |{{rh}}|[[Mexico]] <sup>1</sup>
| |
| |1,676
| |
| |266
| |
| |-
| |
| |11
| |
| |{{rh}}|[[Libya]] (OPEC) <sup>1</sup>
| |
| |1,525
| |
| |242
| |
| |-
| |
| |12
| |
| |{{rh}}|[[Iraq]] (OPEC)
| |
| |1,438
| |
| |229
| |
| |-
| |
| |13
| |
| |{{rh}}|[[Angola]] (OPEC)
| |
| |1,363
| |
| |217
| |
| |-
| |
| |14
| |
| |{{rh}}|[[Kazakhstan]]
| |
| |1,114
| |
| |177
| |
| |-
| |
| |15
| |
| |{{rh}}|[[Canada]] <sup>2</sup>
| |
| |1,071
| |
| |170
| |
| |}
| |
| Source: [http://www.eia.doe.gov/emeu/cabs/topworldtables1_2.htm US Energy Information Administration]
| |
|
| |
|
| <small><sup>1</sup> [[Oil reserves#Countries that have already passed their production peak|peak production already passed in this state]]</small> | | <math>\sigma_z</math> and <math>\sigma_y</math> are functions of the atmospheric stability class (i.e., a measure of the turbulence in the ambient atmosphere) and of the downwind distance to the receptor. The two most important variables affecting the degree of pollutant emission dispersion obtained are the height of the emission source point and the degree of atmospheric turbulence. The more turbulence, the better the degree of dispersion. |
|
| |
|
| <small><sup>2</sup> Canadian statistics are complicated by the fact it is both an importer and exporter of crude oil, and refines large amounts of oil for the U.S. market. It is the leading source of U.S. imports of oil and products, averaging 2.5 MMbbl/d in August 2007. | | Whereas older models rely on stability classes for the determination of <math>\sigma_y</math> and <math>\sigma_z</math>, more recent models increasingly rely on Monin-Obukhov similarity theory to derive these parameters. |
| [http://tonto.eia.doe.gov/dnav/pet/pet_move_impcus_a2_nus_ep00_im0_mbblpd_m.htm].</small>
| |
|
| |
|
| Total world production/consumption (as of 2005) is approximately {{convert|84|Moilbbl/d|m3/d}}.
| | ==Briggs plume rise equations== |
|
| |
|
| See also: [[Organization of Petroleum Exporting Countries]].
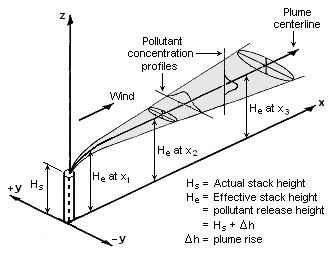
| | The Gaussian air pollutant dispersion equation (discussed above) requires the input of ''H'' which is the pollutant plume's centerline height above ground level. ''H'' is the sum of ''H''<sub>s</sub> (the actual physical height of the pollutant plume's emission source point) plus Δ''H'' (the plume rise due the plume's buoyancy). |
|
| |
|
| ===Import===
| | [[File:Gaussian Plume.png|thumb|right|333px|Visualization of a buoyant Gaussian air pollutant dispersion plume]] |
| [[Image:Oil imports.PNG|thumb|300px|Oil imports by country]] | |
|
| |
|
| In order of net imports in 2006 in thousand [[Barrel (unit)|bbl]]/[[Day|d]] and thousand [[Cubic metre|m³]]/d:
| | To determine Δ''H'', many if not most of the air dispersion models developed between the late 1960s and the early 2000s used what are known as "the Briggs equations." G.A. Briggs first published his plume rise observations and comparisons in 1965.<ref>G.A. Briggs, "A plume rise model compared with observations", ''JAPCA'', 15:433–438, 1965.</ref> In 1968, at a symposium sponsored by CONCAWE (a Dutch organization), he compared many of the plume rise models then available in the literature.<ref>G.A. Briggs, "CONCAWE meeting: discussion of the comparative consequences of different plume rise formulas", ''Atmos. Envir.'', 2:228–232, 1968.</ref> In that same year, Briggs also wrote the section of the publication edited by Slade<ref>D.H. Slade (editor), "Meteorology and atomic energy 1968", Air Resources Laboratory, U.S. Dept. of Commerce, 1968.</ref> dealing with the comparative analyses of plume rise models. That was followed in 1969 by his classical critical review of the entire plume rise literature,<ref>G.A. Briggs, "Plume Rise", ''USAEC Critical Review Series'', 1969.</ref> in which he proposed a set of plume rise equations which have become widely known as "the Briggs equations". Subsequently, Briggs modified his 1969 plume rise equations in 1971 and in 1972.<ref>G.A. Briggs, "Some recent analyses of plume rise observation", ''Proc. Second Internat'l. Clean Air Congress'', Academic Press, New York, 1971.</ref><ref>G.A. Briggs, "Discussion: chimney plumes in neutral and stable surroundings", ''Atmos. Envir.'', 6:507–510, 1972.</ref> |
| {| style="text-align: right;" border="1" cellspacing="0" class="wikitable sortable"
| |
| !#
| |
| !Importing Nation (2006)
| |
| !(10<sup>3</sup>bbl/day)
| |
| !(10<sup>3</sup>m<sup>3</sup>/day)
| |
| |-
| |
| |1
| |
| |{{rh}}|United States <sup>1</sup>
| |
| |12,220
| |
| |1,943
| |
| |-
| |
| |2
| |
| |{{rh}}|Japan
| |
| |5,097
| |
| |810
| |
| |-
| |
| |3
| |
| |{{rh}}|China <sup>2</sup>
| |
| |3,438
| |
| |547
| |
| |-
| |
| |4
| |
| |{{rh}}|Germany
| |
| |2,483
| |
| |395
| |
| |-
| |
| |5
| |
| |{{rh}}|South Korea
| |
| |2,150
| |
| |342
| |
| |-
| |
| |6
| |
| |{{rh}}|France
| |
| |1,893
| |
| |301
| |
| |-
| |
| |7
| |
| |{{rh}}|India
| |
| |1,687
| |
| |268
| |
| |-
| |
| |8
| |
| |{{rh}}|Italy
| |
| |1,558
| |
| |248
| |
| |-
| |
| |9
| |
| |{{rh}}|Spain
| |
| |1,555
| |
| |247
| |
| |-
| |
| |10
| |
| |{{rh}}|Republic of China (Taiwan)
| |
| |942
| |
| |150
| |
| |-
| |
| |11
| |
| |{{rh}}|Netherlands
| |
| |936
| |
| |149
| |
| |-
| |
| |12
| |
| |{{rh}}|Singapore
| |
| |787
| |
| |125
| |
| |-
| |
| |13
| |
| |{{rh}}|Thailand
| |
| |606
| |
| |96
| |
| |-
| |
| |14
| |
| |{{rh}}|Turkey
| |
| |576
| |
| |92
| |
| |-
| |
| |15
| |
| |{{rh}}|Belgium
| |
| |546
| |
| |87
| |
| |}
| |
| Source: [http://www.eia.doe.gov/emeu/cabs/topworldtables1_2.htm US Energy Information Administration]
| |
|
| |
|
| <small><sup>1</sup> [[Oil reserves#Countries that have already passed their production peak|peak production of oil already passed in this state]]</small>
| | Briggs divided air pollution plumes into these four general categories: |
| | * Cold jet plumes in calm ambient air conditions |
| | * Cold jet plumes in windy ambient air conditions |
| | * Hot, buoyant plumes in calm ambient air conditions |
| | * Hot, buoyant plumes in windy ambient air conditions |
|
| |
|
| <small><sup>2</sup> Major oil producer whose production is still increasing</small>
| | Briggs considered the trajectory of cold jet plumes to be dominated by their initial velocity momentum, and the trajectory of hot, buoyant plumes to be dominated by their buoyant momentum to the extent that their initial velocity momentum was relatively unimportant. Although Briggs proposed plume rise equations for each of the above plume categories, '''''it is important to emphasize that "the Briggs equations" which become widely used are those that he proposed for bent-over, hot buoyant plumes'''''. |
|
| |
|
| ===Non-producing consumers===
| | In general, Briggs's equations for bent-over, hot buoyant plumes are based on observations and data involving plumes from typical combustion sources such as the flue gas stacks from steam-generating boilers burning fossil fuels in large power plants. Therefore the stack exit velocities were probably in the range of 20 to 100 ft/s (6 to 30 m/s) with exit temperatures ranging from 250 to 500 °F (120 to 260 °C). |
| Countries whose oil production is 10% or less of their consumption.
| |
|
| |
|
| {| style="text-align: right;" border="1" cellspacing="0" class="wikitable sortable"
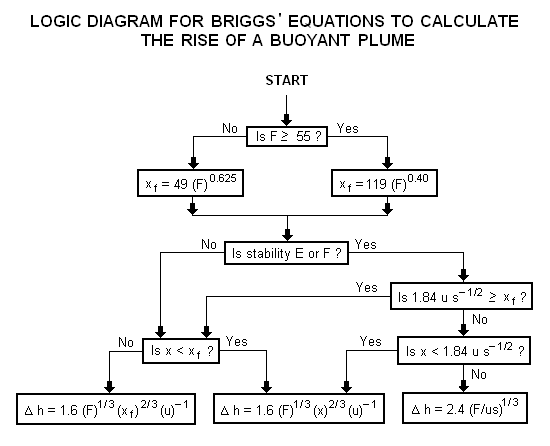
| | A logic diagram for using the Briggs equations<ref name=Beychok/> to obtain the plume rise trajectory of bent-over buoyant plumes is presented below: |
| !#
| | [[Image:BriggsLogic.png|none]] |
| !Consuming Nation
| | :{| border="0" cellpadding="2" |
| !(bbl/day)
| |
| !(m³/day)
| |
| |- | | |- |
| |1 | | |align=right|where: |
| |{{rh}}|Japan
| | | |
| |5,578,000 | |
| |886,831 | |
| |- | | |- |
| |2 | | !align=right| Δh |
| |{{rh}}|Germany | | |align=left|= plume rise, in m |
| |2,677,000
| |
| |425,609
| |
| |- | | |- |
| |3 | | !align=right| F<sup> </sup> <!-- The HTML is needed to line up characters. Do not remove.--> |
| |{{rh}}|South Korea | | |align=left|= buoyancy factor, in m<sup>4</sup>s<sup>−3</sup> |
| |2,061,000
| |
| |327,673
| |
| |- | | |- |
| |4 | | !align=right| x |
| |{{rh}}|France | | |align=left|= downwind distance from plume source, in m |
| |2,060,000
| |
| |327,514
| |
| |- | | |- |
| |5 | | !align=right| x<sub>f</sub> |
| |{{rh}}|Italy | | |align=left|= downwind distance from plume source to point of maximum plume rise, in m |
| |1,874,000
| |
| |297,942
| |
| |- | | |- |
| |6 | | !align=right| u |
| |{{rh}}|Spain | | |align=left|= windspeed at actual stack height, in m/s |
| |1,537,000
| |
| |244,363
| |
| |- | | |- |
| |7 | | !align=right| s<sup> </sup> <!-- The HTML is needed to line up characters. Do not remove.--> |
| |{{rh}}|Netherlands | | |align=left|= stability parameter, in s<sup>−2</sup> |
| |946,700
| |
| |150,513
| |
| |} | | |} |
| Source : [https://www.cia.gov/library/publications/the-world-factbook/rankorder/2175rank.html CIA World Factbook]
| | The above parameters used in the Briggs' equations are discussed in Beychok's book.<ref name=Beychok/> |
|
| |
|
| ==Environmental effects== | | ==References== |
| | | {{reflist}} |
| The presence of oil has significant [[society|social]] and [[environment (biophysical)|environment]]al impacts, from accidents and routine activities such as [[seismology|seismic]] exploration, [[drilling]], and generation of [[pollution|polluting]] wastes, [[greenhouse gas]]es and [[climate change]] not produced by [[renewable energy]].
| |
|
| |
|
| === Extraction === | | == Further reading== |
| Oil extraction is costly and sometimes environmentally damaging, although Dr. [[John Hunt (oceanographer)|John Hunt]] of the [[Woods Hole Oceanographic Institution]] pointed out in a 1981 paper that over 70% of the reserves in the world are associated with visible [[seep|macroseepage]]s, and many oil fields are found due to natural [[seep]]s. Offshore exploration and extraction of oil disturbs the surrounding marine environment.<ref>[http://www.offshore-environment.com/discharges.html Waste discharges during the offshore oil and gas activity] by Stanislave Patin, tr. Elena Cascio</ref> Extraction may involve [[dredging]], which stirs up the [[seabed]], killing the sea plants that marine creatures need to survive. But at the same time, offshore [[oil platform]]s also form micro-habitats for marine creatures.
| |
|
| |
|
| === Oil spills === | | *{{cite book | author=M.R. Beychok| title=Fundamentals Of Stack Gas Dispersion | edition=4th Edition | publisher=author-published | year=2005 | isbn=0-9644588-0-2}} |
|
| |
|
| Crude oil and refined fuel [[Oil spill|spills]] from [[tanker (ship)|tanker ship]] accidents have damaged natural [[ecosystem]]s in [[Alaska]], the [[Galapagos Islands]], [[France]] and many [[List of oil spills|other places]]. The quantity of oil spilled during accidents has ranged from a few hundred tons to several hundred thousand tons (e.g., [[Atlantic Empress]], [[Amoco Cadiz]]). Smaller spills have already proven to have a great impact on ecosystems, such as the [[Exxon Valdez oil spill]]
| | *{{cite book | author=K.B. Schnelle and P.R. Dey| title=Atmospheric Dispersion Modeling Compliance Guide | edition=1st Edition| publisher=McGraw-Hill Professional | year=1999 | isbn=0-07-058059-6}} |
|
| |
|
| Oil spills at sea are generally much more damaging than those on land, since they can spread for hundreds of [[nautical mile]]s in a thin [[oil slick]] which can cover [[beach]]es with a thin coating of oil. This can kill sea birds, mammals, shellfish and other organisms it coats. Oil spills on land are more readily containable if a makeshift earth [[dam]] can be rapidly [[bulldozed]] around the spill site before most of the oil escapes, and land animals can avoid the oil more easily.
| | *{{cite book | author=D.B. Turner| title=Workbook of Atmospheric Dispersion Estimates: An Introduction to Dispersion Modeling | edition=2nd Edition | publisher=CRC Press | year=1994 | isbn=1-56670-023-X}} |
|
| |
|
| Control of oil spills is difficult, requires ad hoc methods, and often a large amount of manpower (picture). The dropping of bombs and incendiary devices from aircraft on the [[Torrey Canyon]] wreck produced poor results;<ref>[[Torrey Canyon#Accident|Torrey Canyon bombing by the Navy and RAF]]</ref> modern techniques would include pumping the oil from the wreck, like in the [[Prestige oil spill]] or the [[Erika (tanker)|Erika]] oil spill.<ref>[http://www.total.com/en/group/news/special_report_erika/erika_measures_total/erika_pumping_cargo_11379.htm Pumping of the Erika cargo]</ref>
| | *{{cite book | author= S.P. Arya| title=Air Pollution Meteorology and Dispersion | edition=1st Edition | publisher=Oxford University Press | year=1998 | isbn=0-19-507398-3}} |
|
| |
|
| | *{{cite book | author=R. Barrat| title=Atmospheric Dispersion Modelling | edition=1st Edition | publisher=Earthscan Publications | year=2001 | isbn=1-85383-642-7}} |
|
| |
|
| | *{{cite book | author=S.R. Hanna and R.E. Britter| title=Wind Flow and Vapor Cloud Dispersion at Industrial and Urban Sites | edition=1st Edition | publisher=Wiley-American Institute of Chemical Engineers | year=2002 | isbn=0-8169-0863-X}} |
|
| |
|
| ==Future of petroleum production==
| | *{{cite book | author=P. Zannetti| title=Air pollution modeling : theories, computational methods, and available software | edition= | publisher= Van Nostrand Reinhold | year=1990 | isbn=0-442-30805-1 }} |
| | |
| ''[[USA Today]]'' news reported in 2004 that there were 40 years of petroleum left in the ground. As similar statements have been made in the 40 previous years, it hardly carries the complex situation.
| |
| | |
| Consumption in the twentieth century has been abundantly pushed by automobile growth ; the [[1980s oil glut|1985-2003 oil glut]] even fuelled the sales of low economy vehicles (SUVs) in [[OECD]] countries. In 2008, the economic crisis seems to have some impact on the sales of such vehicles ; still, the 2008 oil consumption shows a small increase. The [[BRIC]] countries might also kick in, as China briefly was the first automobile market in december 2009<ref name=China-market>
| |
| {{cite web | |
| |url=http://news.bbc.co.uk/2/hi/business/7879372.stm
| |
| |title=China's car industry overtakes US
| |
| |author=Chris Hogg
| |
| |date=2009-02-10
| |
| }}</ref> . The immediate outlook still hints upwards.
| |
| In the long term, uncertainties loiter ; the [[OPEC]] believes that the [[OECD]] countries will push low consumption policies at some point in the future ; when that happens, it will definitely curb the oil sales, and both [[OPEC]] and [[Energy Information Administration|EIA]] kept lowering their 2020 consumption estimates during the past 5 years <ref name="OPEC-Publications, fig 1.19 P48">
| |
| {{cite web
| |
| |url=http://www.opec.org/library/World%20Oil%20Outlook/pdf/WOO2008.pdf
| |
| |title=World Oil Outlook 2008
| |
| |author=OPEC Secretariat
| |
| |date=2008
| |
| }}</ref>. Oil products are more and more in competition with alternative sources, mainly coal and natural gas, both cheaper sources.
| |
| | |
| Production will also face an increasingly complex situation ; while OPEC countries still have large reserves at low production prices, newly found reservoirs often lead to higher prices ; offshore giants such as [[Tupi]], Guara and [[Tiber oilfield|Tiber]] demand high investments and ever-increasing technological abilities. Subsalt reservoirs such as Tupi were unknown in the twentieth century, mainly because the industry was unable to probe them. [[Enhanced Oil Recovery]] (EOR) techniques (example : [[Daqing Field|DaQing]], China <ref name=DaQing>
| |
| {{cite web
| |
| |url=http://en.ce.cn/Insight/200610/16/t20061016_8980162.shtml
| |
| |title=Daqing Oilfield rejuvenated by virtue of technology
| |
| |author=Ni Weiling
| |
| |date=2006-10-16
| |
| }}</ref> ) will continue to play a major role in increasing the world's recoverable oil.
| |
| | |
| ===Hubbert peak theory===
| |
| {{main|Peak oil|Hubbert peak theory}}
| |
| The [[Hubbert peak theory]] (also known as [[peak oil]]) posits that future petroleum production (whether for individual oil wells, entire oil fields, whole countries, or worldwide production) will eventually peak and then decline at a similar rate to the rate of increase before the peak as these reserves are exhausted. The peak of oil discoveries was in 1965, and oil production per year has surpassed oil discoveries every year since 1980.<ref name=campbell1222000>
| |
| {{cite web
| |
| |url=http://energycrisis.org/de/lecture.html
| |
| |title=Peak Oil Presentation at the Technical University of Clausthal
| |
| |author=Campbell CJ
| |
| |date=2000-12
| |
| }}</ref>
| |
|
| |
| | |
| Controversy surrounds predictions of the timing of the global peak, as these predictions are dependent on the past production and discovery data used in the calculation as well as how unconventional reserves are considered. Also, these predictions do not take into account outside elements such as the current economic crisis (2008). Also, many Peak Oil promoters proposed many different dates, some of them passed already .
| |
| | |
| It is difficult to predict the [[peak oil|oil peak]] in any given region, due to the lack of knowledge and/or transparency in [[accounting]] of global oil reserves.<ref>[http://www.iags.org/n0331043.htm New study raises doubts about Saudi oil reserves]</ref> Based on available production data, proponents have previously predicted the peak for the world to be in years 1989, 1995, or 1995-2000. Some of these predictions date from before the recession of the early 1980s, and the consequent reduction in global consumption, the effect of which was to delay the date of any peak by several years. Just as the 1971 U.S. peak in oil production was only clearly recognized after the fact, a peak in world production will be difficult to discern until production clearly drops off.
| |
| | |
| ==References==
| |
| {{reflist}}
| |



![{\displaystyle \exp \;[-\,y^{2}/\,(2\;\sigma _{y}^{2}\;)\;]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e8925630e3b8de6be025fb258aa809189a852082)



![{\displaystyle \;\exp \;[-\,(z-H)^{2}/\,(2\;\sigma _{z}^{2}\;)\;]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/32be81e17b8659fb72ef85e886a95c57401160bd)

![{\displaystyle \;\exp \;[-\,(z+H)^{2}/\,(2\;\sigma _{z}^{2}\;)\;]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b8359e355bf985dc7714abecb7a250155e07b62f)

![{\displaystyle \sum _{m=1}^{\infty }\;{\big \{}\exp \;[-\,(z-H-2mL)^{2}/\,(2\;\sigma _{z}^{2}\;)\;]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/37cb296347f56a0618c59d247cbf00125fc3de8c)
![{\displaystyle +\,\exp \;[-\,(z+H+2mL)^{2}/\,(2\;\sigma _{z}^{2}\;)\;]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8546ac2627ada5bfebad51a692c902206a5efa2c)
![{\displaystyle +\,\exp \;[-\,(z+H-2mL)^{2}/\,(2\;\sigma _{z}^{2}\;)\;]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1fe6a89e17f16835d86e8d9a8971a74db46d1e4b)
![{\displaystyle +\,\exp \;[-\,(z-H+2mL)^{2}/\,(2\;\sigma _{z}^{2}\;)\;]{\big \}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6ff94c96673914895d6e0a6d2643dca0e9e4bc77)