Cytoskeleton: Difference between revisions
imported>Gareth Leng No edit summary |
mNo edit summary |
||
| (20 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
The '''cytoskeleton''' is a | The '''cytoskeleton''' is a structural "[[scaffold]]" or "[[skeleton]]" that is present inside all [[eukaryotic]] cells. This dynamic, three dimensional structure fills the cytoplasms of cells; it maintains their shape, and enables them to move, using structures such as [[flagellum|flagella]] and [[cilium|cilia]]. It also has important roles in the intracellular transport of [[vesicle (biology)|vesicle]]s and organelles) and in cell division. | ||
== | ==Eukaryotic cells== | ||
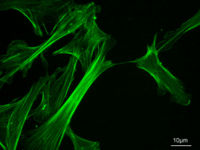
[[Image:MEF_microfilaments.jpg|thumb|right|200px|Actin cytoskeleton of [[mus_musculus|mouse]] [[embryo]] [[fibroblast]]s, stained with [[phalloidin]]]] | [[Image:MEF_microfilaments.jpg|thumb|right|200px|Actin cytoskeleton of [[mus_musculus|mouse]] [[embryo]] [[fibroblast]]s, stained with [[phalloidin]]]] | ||
[[Eukaryotic]] cells contain three kinds of cytoskeletal filaments | [[Eukaryotic]] cells contain three kinds of cytoskeletal filaments: | ||
*'''Microfilaments''', which are about 7 nm in diameter, are composed of two [[actin]] chains oriented in an helicoidal shape. Actin is a contractile protein, and is the most abundant protein in cells. In muscle cells, the association of microfilaments with another protein, [[myosin]], is responsible for muscle contraction. Microfilaments can also carry out cellular movements including gliding, contraction, and cytokinesis. They are mostly concentrated just beneath the plasma membrane, as they keep cellular shape, form cytoplasmatic protuberances (like [[pseudopod|pseudopodia]] and [[microvillus| | |||
'' | microvilli]]), and participate in some cell-to-cell or cell-to-matrix junctions and in the [[transduction]] of signals. | ||
*'''[[Intermediate filament]]s''', 8-11 nanometers in diameter, are the more stable (strongly bound) and heterogeneous constituents of the cytoskeleton; they provide the tensile strength of a cell, and determine its internal structure; for example, they are structural components of the [[nuclear envelope]] and of the [[sarcomere]]s. They also participate in some cell-cell and cell-matrix junctions. Many intermediate filaments are made of [[vimentin]]s. Others, in cells of the [[skin]] [[hair]] and [[nails]], are made of [[keratin]]. Some filaments, made of [[laminin]], give structural support to the nuclear envelope.<ref>Zhong Z ''et al.'' (2010) Beyond lamins other structural components of the nucleoskeleton ''Methods Cell Biol'' 98:97-119 PMID 20816232</ref> | |||
*'''[[Microtubule]]s''' are hollow cylinders of about 25 nm in diameter, formed by 13 protofilaments which are polymers of alpha and beta [[tubulin]]. Microtubules act as a scaffold to determine cell shape, and they provide "tracks" which cell organelles and vesicles move along. Microtubules also form the spindle fibers for separating chromosomes during mitosis. When arranged in geometric patterns inside flagella and cilia, they are used for locomotion. They have a very dynamic behaviour, binding [[Guanosine triphosphate|GTP]] for polymerization. They are organized by the [[centrosome]]. <ref>Bereiter-Hahn J, Jendrach M (2010) | |||
Mitochondrial dynamics ''Int Rev Cell Mol Biol'' 284:1-65. PMID 20875628</ref> | |||
'' | |||
In, for example, epithelial (skin) cells of the intestine, microfilaments that project into the [[villus|villi]], give shape to the cell surface; microtubules project from the centrosome to the cell periphery; and intermediate filaments connect adjacent cells through [[desmosome]]s. | |||
The cytoskeleton of mammalian [[Sertoli cell]]s is one of the most elaborate that has been described. Actin filaments, vimentin-type intermediate filaments and microtubules all have distinct patterns of distribution that change during [[spermatogenesis]]. Actin filaments are concentrated in ectoplasmic specializations that are involved in intercellular adhesion, and at complexes involved with junction internalization during sperm release and movement of spermatocytes between neighbouring Sertoli cells. Intermediate filaments are in a perinuclear network which has peripheral extensions to desmosome-like junctions with adjacent cells and to small attachments to the basal lamina, and are thought to have a role in maintaining tissue integrity when the epithelium is mechanically stressed. Microtubules are involved in maintaining the columnar shape of Sertoli cells, with transporting organelles in the cytoplasm, with secreting seminiferous tubule fluid, and in intercellular communication. <ref>Vogl AW ''et al.'' (2008) The Sertoli cell cytoskeleton ''Adv Exp Med Biol'' 636:186-211 PMID 19856169</ref> | |||
=== | ==Prokaryote cells== | ||
< | The cytoskeleton was once believed to be a feature only of [[eukaryote|eukaryotic]] cells, but [[homology (biology)|homologues]] of the major proteins of the eukaryotic cytoskeleton have been found in [[prokaryotes]].<ref>{{cite journal |author=Shih YL, Rothfield L |title=The bacterial cytoskeleton |journal=Microbiol Mol Biol Rev|volume=70 |pages=729–54 |year=2006 |pmid=16959967}}</ref> <ref>{{cite journal |author=Michie KA, Löwe J |title=Dynamic filaments of the bacterial cytoskeleton |journal=Annu Rev Biochem |volume=75 |pages=467–92 |year=2006 |pmid=16756499 }}</ref>Cabeen MT, Jacobs-Wagner C (2010) The bacterial cytoskeleton ''Annu Rev Genet'' 44:365-92</ref> | ||
[[ | [[FtsZ]] was the first of these to be identified. Like tubulin, FtsZ forms filaments in the presence of [[Guanosine triphosphate|GTP]], but these filaments do not group into tubules. During [[cell division]], FtsZ is the first protein to move to the division site, and is essential for recruiting other proteins that produce a new [[cell wall]] between the dividing cells. | ||
Prokaryotic actin-like proteins, such as [[MreB]], are involved in maintaining cell shape. All non-spherical bacteria have [[gene]]s that encode actin-like proteins; these proteins form a helical network beneath the cell membrane that guides the proteins involved in cell wall [[biosynthesis]]. | |||
Some [[plasmid]]s encode a partitioning system that involves an actin-like protein [[ParM]]. Filaments of ParM exhibit [[dynamic instability]], and may partition plasmid DNA into the dividing daughter cells by a mechanism [[Analogy (biology)|like]] that used by microtubules during eukaryotic [[mitosis]]. | |||
The bacterium ''[[Caulobacter crescentus]]'' expresses a protein, [[crescentin]], that is related to the intermediate filaments of eukaryotic cells. Crescentin is also involved in maintaining cell shape, but how it does this is unclear. | |||
==References== | |||
<references/>[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 4 August 2024
The cytoskeleton is a structural "scaffold" or "skeleton" that is present inside all eukaryotic cells. This dynamic, three dimensional structure fills the cytoplasms of cells; it maintains their shape, and enables them to move, using structures such as flagella and cilia. It also has important roles in the intracellular transport of vesicles and organelles) and in cell division.
Eukaryotic cells
Eukaryotic cells contain three kinds of cytoskeletal filaments:
- Microfilaments, which are about 7 nm in diameter, are composed of two actin chains oriented in an helicoidal shape. Actin is a contractile protein, and is the most abundant protein in cells. In muscle cells, the association of microfilaments with another protein, myosin, is responsible for muscle contraction. Microfilaments can also carry out cellular movements including gliding, contraction, and cytokinesis. They are mostly concentrated just beneath the plasma membrane, as they keep cellular shape, form cytoplasmatic protuberances (like pseudopodia and microvilli), and participate in some cell-to-cell or cell-to-matrix junctions and in the transduction of signals.
- Intermediate filaments, 8-11 nanometers in diameter, are the more stable (strongly bound) and heterogeneous constituents of the cytoskeleton; they provide the tensile strength of a cell, and determine its internal structure; for example, they are structural components of the nuclear envelope and of the sarcomeres. They also participate in some cell-cell and cell-matrix junctions. Many intermediate filaments are made of vimentins. Others, in cells of the skin hair and nails, are made of keratin. Some filaments, made of laminin, give structural support to the nuclear envelope.[1]
- Microtubules are hollow cylinders of about 25 nm in diameter, formed by 13 protofilaments which are polymers of alpha and beta tubulin. Microtubules act as a scaffold to determine cell shape, and they provide "tracks" which cell organelles and vesicles move along. Microtubules also form the spindle fibers for separating chromosomes during mitosis. When arranged in geometric patterns inside flagella and cilia, they are used for locomotion. They have a very dynamic behaviour, binding GTP for polymerization. They are organized by the centrosome. [2]
In, for example, epithelial (skin) cells of the intestine, microfilaments that project into the villi, give shape to the cell surface; microtubules project from the centrosome to the cell periphery; and intermediate filaments connect adjacent cells through desmosomes.
The cytoskeleton of mammalian Sertoli cells is one of the most elaborate that has been described. Actin filaments, vimentin-type intermediate filaments and microtubules all have distinct patterns of distribution that change during spermatogenesis. Actin filaments are concentrated in ectoplasmic specializations that are involved in intercellular adhesion, and at complexes involved with junction internalization during sperm release and movement of spermatocytes between neighbouring Sertoli cells. Intermediate filaments are in a perinuclear network which has peripheral extensions to desmosome-like junctions with adjacent cells and to small attachments to the basal lamina, and are thought to have a role in maintaining tissue integrity when the epithelium is mechanically stressed. Microtubules are involved in maintaining the columnar shape of Sertoli cells, with transporting organelles in the cytoplasm, with secreting seminiferous tubule fluid, and in intercellular communication. [3]
Prokaryote cells
The cytoskeleton was once believed to be a feature only of eukaryotic cells, but homologues of the major proteins of the eukaryotic cytoskeleton have been found in prokaryotes.[4] [5]Cabeen MT, Jacobs-Wagner C (2010) The bacterial cytoskeleton Annu Rev Genet 44:365-92</ref> FtsZ was the first of these to be identified. Like tubulin, FtsZ forms filaments in the presence of GTP, but these filaments do not group into tubules. During cell division, FtsZ is the first protein to move to the division site, and is essential for recruiting other proteins that produce a new cell wall between the dividing cells.
Prokaryotic actin-like proteins, such as MreB, are involved in maintaining cell shape. All non-spherical bacteria have genes that encode actin-like proteins; these proteins form a helical network beneath the cell membrane that guides the proteins involved in cell wall biosynthesis.
Some plasmids encode a partitioning system that involves an actin-like protein ParM. Filaments of ParM exhibit dynamic instability, and may partition plasmid DNA into the dividing daughter cells by a mechanism like that used by microtubules during eukaryotic mitosis.
The bacterium Caulobacter crescentus expresses a protein, crescentin, that is related to the intermediate filaments of eukaryotic cells. Crescentin is also involved in maintaining cell shape, but how it does this is unclear.
References
- ↑ Zhong Z et al. (2010) Beyond lamins other structural components of the nucleoskeleton Methods Cell Biol 98:97-119 PMID 20816232
- ↑ Bereiter-Hahn J, Jendrach M (2010) Mitochondrial dynamics Int Rev Cell Mol Biol 284:1-65. PMID 20875628
- ↑ Vogl AW et al. (2008) The Sertoli cell cytoskeleton Adv Exp Med Biol 636:186-211 PMID 19856169
- ↑ Shih YL, Rothfield L (2006). "The bacterial cytoskeleton". Microbiol Mol Biol Rev 70: 729–54. PMID 16959967.
- ↑ Michie KA, Löwe J (2006). "Dynamic filaments of the bacterial cytoskeleton". Annu Rev Biochem 75: 467–92. PMID 16756499.