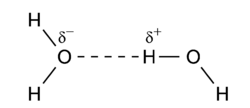Intermolecular forces: Difference between revisions
imported>Paul Wormer |
mNo edit summary |
||
| (99 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{subpages}} | ||
In [[physics]], [[chemistry]], and [[biology]], '''intermolecular forces''' are forces that act between stable [[molecule]]s or between functional groups of [[macromolecule]]s. | : ''See [[Van der Waals forces]] for a brief overview.'' | ||
In [[physics]], [[chemistry]], and [[biology]], '''intermolecular forces''' are forces that act between stable [[molecule]]s or between functional groups of [[macromolecule]]s. Sometimes they are referred to as [[Van der Waals forces]], although some texts apply the latter term to a specific subset of intermolecular forces. | |||
Intermolecular forces are [[non-covalent]] and give rise to bonding energies of a few kcal/mol or less, i.e., they are generally much weaker than [[chemical bonding]] (covalent) forces. Nevertheless, intermolecular forces are the cause of a wide range of physical, chemical, and biological phenomena. For instance, they are responsible for the deviation from the [[ideal gas law]] for real gases, determine the [[tertiary structure]] of [[macromolecules]], and explain why some relatively light molecules are liquid or solid at normal temperature and pressure. | |||
{{TOC|left}} | |||
The sum of long and short range forces gives rise to a minimum, referred to as ''Van der Waals minimum''. The position and depth of the Van der Waals minimum depends on distance and mutual orientation of the molecules. | In general one distinguishes short- and long-range intermolecular forces. As will be explained in this article, the former are mainly due to the intermolecular [[electron exchange|exchange of electrons]], a phenomenon closely related to the quantum mechanical [[Pauli principle]]. Short-range forces fall off exponentially as a function of intermolecular distance ''R'' and are repulsive for interacting stable (closed-shell) moieties. In chemistry they are well known, because they give rise to [[steric effects|steric hindrance]], also known as Born or Pauli repulsion. | ||
The long-range forces, on the other hand, fall off with inverse powers of the distance, ''R''<sup> −''n''</sup>, typically 3 ≤ ''n'' ≤ 10, and are mostly attractive. They are partly caused by interaction between permanent [[molecular dipole]]s and higher [[multipole]]s, by induction effects, and by a quantum mechanical effect for which [[Fritz London]] coined the name dispersion effect. | |||
The sum of the attractive long-range and repulsive short-range forces gives rise to a minimum, referred to as ''Van der Waals minimum''. The position and depth of the Van der Waals minimum depends on distance and mutual orientation of the molecules. | |||
==Observation versus computation== | |||
In general, a [[force]] is minus the [[gradient]] (set of derivatives with respect to coordinates) of a [[potential]]. Therefore, it is common to concentrate on the intermolecular ''potential'' rather than on its derivatives—the intermolecular ''force''. An intermolecular potential—a scalar function of the positions of the nuclei constituting a molecular complex—''cannot'' be measured directly. Experimental information is obtained from measurable properties that depend on the intermolecular potential. The standard example is the pressure-volume-temperature (''P-V-T'') dependence of a [[Van der Waals equation|van der Waals fluid]]. After measurement of the ''P-V-T'' relation, the two parameters in the van der Waals equation can be determined and these parameters tell something about the interaction in a pair of molecules constituting the fluid. Clearly, by a simple extension the same holds for more elaborate equations of state, i.e., for multiparameter ''P-V-T'' relations. | |||
Other examples of situations where the intermolecular potential plays a role, are transport properties of fluids, molecular collision cross-sections, spectra of molecular complexes (dimers, trimers, …), and structures of molecular solids. In all these cases the observed properties depend on the positions of the nuclei via the intermolecular potential. The dependence of the property on the potential can be simple, as in the example of a van der Waals gas, or through a set of elaborate classical or quantum mechanical equations. However, all these observable properties have in common that the road backward from experimental values to the intermolecular potential is non-unique. Very different potentials may predict the same observables. Before the advent of computers, it was common to assume a simple model potential, containing a few adjustable parameters, and to modify these parameters so that an optimum match with the observations was found. Almost always these potentials lacked transferability, the potential applied to other properties of the same molecular complex fails in predicting properties other than those considered in the matching procedure. | |||
In the last decade of the 20th century it became possible to compute the interaction energy of two (fairly small) molecules ''ab initio'' (from the beginning), that is, by solving the [[Schrödinger equation]] of the interacting molecules without any empirical input other than nuclear charges. Of course, many approximations have to be made along the way, but all of these are well investigated and their effects are known quantitatively. At present, therefore, the most reliable source of quantitative knowledge of intermolecular potentials is by ''ab initio'' computation. Once the potential has been calculated, one can go to the observable properties. This second step involves the solution of the equation of motion of the nuclei with the computed potential as input. The equation may be classical, associated with the names of [[Newton]], [[Lagrange]], or [[Hamilton]], or the equation may be the quantum mechanical Schrödinger equation. The choice depends on whether or not quantum effects are important for the property under consideration. | |||
The present article describes the most important effects that contribute to the intermolecular potential and that must be included in the ''ab initio'' computation of the potential. | |||
==Historical background and the need for quantum mechanics== | |||
Before the advent of [[quantum mechanics]] (middle of the 1920s), the origin of intermolecular forces was unknown.<ref> See H. Margenau and N. R. Kestner, ''Theory of Intermolecular Forces'', Pergamon Press, Oxford, (1960) for an overview of the failed pre-quantum mechanical efforts in finding an explanation.</ref> Especially the causes of hard sphere repulsion between molecules, postulated by [[Van der Waals]], and the possibility of the [[liquefaction of gases|liquefaction]] of [[noble gas]]es, first observed (1908) by [[Heike Kamerlingh Onnes]] for helium, were very puzzling, as these effects could not be explained at all by classical [[electromagnetism| electromagnetic]] theory or by the "old" quantum theory. Soon after the formulation of the "new" [[quantum mechanics]] the pieces of the puzzle fell together and all open questions regarding intermolecular forces were answered, first by S.C. Wang and then more completely and thoroughly by [[Fritz London]]. | |||
One of the more important effects is the [[dispersion interaction]], a term coined in 1930 by London. This attractive interaction is present for all atom- and molecule-pairs, even for [[noble gas]] atoms. In fact, the dispersion force explains why helium gas can be liquefied. The dispersion force is often explained as due to an interaction between ''instantaneous [[molecular dipole|electric dipole]]s''. The term "instantaneous" suggests time dependence and some textbooks add that the instantaneous dipole-dipole interaction can be described ''classically'', i.e., without quantum mechanics. London (also in 1930) gave a model of two oscillating [[electric dipole]]s interacting through an electrostatic [[Coulomb's law|Coulomb force]] and showed that time-''in''dependent quantum mechanics describes this attraction. Moreover, London emphasized that the zero-point motion of the oscillators, a typical quantum mechanical effect related to [[Heisenberg's uncertainty relation]], is a necessary part of the explanation. Neither classical theory, nor the "old" Planck-Bohr quantum theory accounts for zero-point motion. In the article [[dispersion interaction]] London's oscillating dipole model is discussed in more detail. | |||
The present article will sketch the theory of dispersion, repulsion, and other intermolecular forces and by necessity will invoke a few quantum mechanical concepts. | |||
Effects like dipole-dipole interaction and dipole-induced dipole interaction can be accounted for by classical theories provided the values of [[molecular dipole]]s and molecular [[polarizability|polarizabilities]] are known. However, classical theory cannot explain why a molecule is a stable charge distribution, i.e., why the negative [[electron]]s do not fall down on the positive [[nucleus (atom)|nuclei]] while losing their energy by emitting [[electromagnetic radiation]]. This also means that classical theory cannot predict the size of a molecular dipole or of a molecular polarizability. Quantum mechanics, on the other hand, does not only account ''qualitatively'' for all observed effects, but also ''quantitatively''. As pointed out above, today's computer calculations, based on quantum physics, form a reliable source of quantitative information about intermolecular forces. | |||
Finally, it is of interest to point out that for those cases where a classical explanation exists (notably for electrostatic and induction forces) the quantum and the classical descriptions agree. This will be shown below. | |||
==General theory== | ==General theory== | ||
<font style = "font-size: 80%; font-style: oblique; font-weight: bolder" > From here on this article presupposes some knowledge of quantum mechanics, in particular the concept of [[antisymmetric wave function]]s and elementary [[perturbation theory]].</font> | |||
The quantum mechanical basis for the majority of intermolecular effects is contained in a nonrelativistic energy operator, the [[molecular Hamiltonian]]. This operator consists only of kinetic energies and Coulomb interactions. Invoking the [[Born-Oppenheimer approximation|Born-Oppenheimer (BO) approximation]], one considers the electronic (clamped nuclei) Hamilton operator only. The BO approximation allows the definition of an intermolecular potential energy. It is only by virtue of the BO approximation that one can consider the potential and its gradients with respect to different intermolecular degrees of freedom—the intermolecular forces. | |||
For very long intermolecular distances the retardation of the Coulomb force (first considered in 1948 for intermolecular forces by [[Hendrik Casimir]] and [[Dirk Polder]]) may have to be included. Sometimes, e.g., for interacting [[paramagnetism|paramagnetic]] or electronically [[excited state|excited molecules]], electronic [[Spin (physics)|spin]] and other magnetic effects may play a role. In this article, however, retardation and magnetic effects will not be considered. | |||
The books<ref name="Stone">A. J. Stone, ''The Theory of Intermolecular Forces'', 1996, Clarendon Press, Oxford</ref><ref name="Kaplan">I. G. Kaplan, ''Intermolecular Interactions'', Wiley, Hoboken NJ (2006)</ref> may be consulted for modern and thorough expositions of the theory of intermolecular forces. | |||
It is common to distinguish four fundamental interactions, see e.g., Refs. <ref name = "Stone"/><ref name = "Kaplan"/><ref name = "Jeziorski">B. Jeziorski, R. Moszynski, and K. Szalewicz, ''Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals complexes'', Chemical Reviews, vol. '''94''', pp. 1887-1930 (1994). [http://dx.doi.org/10.1021/cr00031a008 doi].</ref><ref name = "Szalewicz"> K. Szalewicz, K. Patkowski, and B. Jeziorski, ''Intermolecular interactions via perturbation theory: from diatoms to biomolecules'', in: Structure and Bonding, vol. '''116''', pp. 43-117 (2005). </ref> | |||
*exchange | *exchange | ||
*electrostatic | *electrostatic | ||
| Line 26: | Line 54: | ||
V^{AB} \equiv H^{AB} - H^{A}- H^{B} = \sum_{i\in A} \sum_{j \in B} \frac{q_i q_j}{r_{ij}}, | V^{AB} \equiv H^{AB} - H^{A}- H^{B} = \sum_{i\in A} \sum_{j \in B} \frac{q_i q_j}{r_{ij}}, | ||
</math> | </math> | ||
where ''q''<sub> ''i''</sub> indicates the charge (in units ''e'' of [[elementary charge]]) of a particle of monomer ''A''; ''q''<sub> ''j''</sub> belongs to monomer ''B''. | where ''q''<sub> ''i''</sub> indicates the charge (in units ''e'' of [[elementary charge]]) of a particle of monomer ''A''; ''q''<sub> ''j''</sub> belongs to monomer ''B''. In the case of electrons ''q'' = −1, while a nucleus has ''q'' equal to its [[atomic number]] ''Z''. The quantity ''r''<sub> ''ij''</sub> is the distance between particle ''i'' and particle ''j''. | ||
Perturbation theory is based on expansions of perturbed states in terms of unperturbed states (eigenstates of ''H''<sup>(0)</sup>). In the present case the unperturbed states are products | Perturbation theory is based on expansions of perturbed states in terms of unperturbed states (eigenstates of ''H''<sup>(0)</sup> ). In the present case the unperturbed states are products | ||
:<math> | :<math> | ||
\Phi_n^A \Phi_m^B\quad \hbox{with}\quad H^A \Phi_n^A = E_n^A\Phi_n^A\quad\hbox{and}\quad H^B \Phi_m^B = E_m^B\Phi_m^B | \Phi_n^A \Phi_m^B\quad \hbox{with}\quad H^A \Phi_n^A = E_n^A\Phi_n^A\quad\hbox{and}\quad H^B \Phi_m^B = E_m^B\Phi_m^B | ||
</math> | </math> | ||
'''Note on units:''' As is very common in quantum mechanical expositions, [[Gaussian units]] are applied here, that is, there appears no factor 1/(4πε<sub>0</sub>) in the Coulomb interaction. In this article the equations are further simplified by application of [[atomic units]]. Atomic units are derived from Gaussian units by putting [[Planck's constant|Planck's reduced constant]] <math>\hbar\,</math> and the [[elementary charge|charge]] and mass of the electron equal to unity. | |||
====Supermolecular approach==== | ====Supermolecular approach==== | ||
The early theoretical work on intermolecular forces was invariably based on RS-PT and its antisymmetrized variants. However, since the beginning of the 1990s it has become possible to apply standard [[Computational chemistry#Electronic structure|quantum chemical method]]s to pairs of molecules. This approach is referred to as the ''supermolecule method''. In order to obtain reliable results one must include [[electronic correlation]] in the supermolecule method (without it dispersion is not accounted for at all), and take care of the ''basis set superposition error''. This is the effect that the atomic orbital basis of one molecule improves the basis of the other. Since this improvement is distance dependent, it gives easily rise to artifacts. | The early theoretical work on intermolecular forces was invariably based on RS-PT and its antisymmetrized variants. However, since the beginning of the 1990s it has become possible to apply standard [[Computational chemistry#Electronic structure|quantum chemical method]]s to pairs of molecules. This approach is referred to as the ''supermolecule method''. In order to obtain reliable results one must include [[electronic correlation]] in the supermolecule method (without it dispersion is not accounted for at all), and take care of the ''basis set superposition error''. This is the effect that the atomic orbital basis of one molecule improves the basis of the other. Since this improvement is distance dependent, it gives easily rise to artifacts. | ||
Supermolecule calculations must be performed with very high precision, because the problem, known as ''weighing the captain'', arises here. First | Supermolecule calculations must be performed with very high precision, because the problem, known as ''weighing the captain'', arises here. First one weighs the ship with the captain aboard (total energy of molecules in interaction) and then the ship is weighed with the captain ashore (total energy of molecules at an infinite distance apart); the difference gives the captain's weight. This analogy is due to the late [[Charles Coulson]]. The analogy is based on the fact that the total energy of molecules is six to seven orders of magnitude larger than a typical intermolecular interaction. That is, the significant digits in the results of supermolecule calculations start to appear beyond the sixth or seventh decimal place. | ||
A disadvantage of the supermolecule method is that it yields the interaction as a lump sum. It does not give an interaction energy separated in the four fundamental contributions mentioned above. Therefore, | A disadvantage of the supermolecule method is that it yields the interaction as a lump sum. It does not give an interaction energy separated in the four fundamental contributions mentioned above. Therefore, the supermolecule will not be discussed method any further in this article. | ||
== | ==Electron exchange== | ||
The monomer functions Φ<sub>''n''</sub><sup>''A''</sup> and Φ<sub>''m''</sub><sup>''B''</sup> are antisymmetric under permutation of electron coordinates (i.e., they satisfy the [[Pauli principle]]), but the product states are not antisymmetric under intermolecular exchange of the electrons. An obvious way to proceed would be to introduce the [[Antisymmetrizer#Intermolecular antisymmetrizer|intermolecular antisymmetrizer]] <math>\tilde{\mathcal{A}}^{AB}</math>. But, as already noticed in 1930 by Eisenschitz and London,<ref name="Eisenschitz">R. Eisenschitz and F. London, Zeitschrift für Physik, vol. 60, p. 491 (1930). English translations in H. Hettema, ''Quantum Chemistry, Classic Scientific Papers,'' World Scientific, Singapore (2000), p. 336.</ref> this causes two | The monomer functions Φ<sub>''n''</sub><sup>''A''</sup> and Φ<sub>''m''</sub><sup>''B''</sup> are antisymmetric under permutation of electron coordinates (i.e., they satisfy the [[Pauli principle]]), but the product states are not antisymmetric under intermolecular exchange of the electrons. An obvious way to proceed would be to introduce the [[Antisymmetrizer#Intermolecular antisymmetrizer|intermolecular antisymmetrizer]] <math>\tilde{\mathcal{A}}^{AB}</math>. But, as already noticed in 1930 by Eisenschitz and London,<ref name="Eisenschitz">R. Eisenschitz and F. London, ''Über das Verhältnis der van der Waalsschen Kräfte zu den homöopolaren Bindungskräften'', (On the relation of the Van der Waals forces to the homopolar forces of bonding), Zeitschrift für Physik, vol. 60, p. 491 (1930). [http://dx.doi.org/10.1007/BF01341258 doi] English translations in H. Hettema, ''Quantum Chemistry, Classic Scientific Papers,'' World Scientific, Singapore (2000), p. 336.</ref> this causes two problems. In the first place the antisymmetrized unperturbed states are no longer eigenfunctions of ''H''<sup>(0)</sup>, which follows from the non-commutation | ||
:<math> | :<math> | ||
\big[ \tilde{\mathcal{A}}^{AB}, H^{(0)}\big] \ne 0 . | \big[ \tilde{\mathcal{A}}^{AB}, H^{(0)}\big] \equiv \tilde{\mathcal{A}}^{AB} H^{(0)} - H^{(0)}\tilde{\mathcal{A}}^{AB} \ne 0 . | ||
</math> | </math> | ||
This non-commutation is due to the fact that in ''H''<sup>(0)</sup> the electrons are assigned to a respective molecule, that is, the unperturbed Hamiltonian ''H''<sup>(0)</sup> is not symmetric in all electrons. The total Hamiltonian ''H''<sup>AB</sup> does contain ''all'' electrons symmetrically, which means that ''H''<sup>AB</sup> is invariant under ''all'' permutations and commutes with the intermolecular antisymmetrizer. One can express this by stating that in ''H''<sup>AB</sup> all electrons are indistinguishable, as they must be, whereas in ''H''<sup>(0)</sup> they are only partly indistinguishable. Studies have shown<ref> K. Patkowski, T. Korona, and B. Jeziorski, | |||
''Convergence Behavior of the Symmetry-Adapted Perturbation Theory for States Submerged in Pauli Forbidden Continuum'', J. Chem. Phys. vol. '''115''', pp. 1137-1152 (2001).</ref> that the spectra (sets of energy eigenvalues) of ''H''<sup>(0)</sup> and ''H''<sup>AB</sup> are dramatically different. This means that the effect of the perturbation ''V''<sup>AB</sup> is strong, even at large ''R''. Conventional Rayleigh-Schrödinger perturbation theory is not capable to cope with this situation. | |||
A second problem is that the projected excited states | |||
:<math> | :<math> | ||
\tilde{\mathcal{A}}^{AB} \Phi^A_n \Phi^B_m | \tilde{\mathcal{A}}^{AB} \Phi^A_n \Phi^B_m | ||
</math> | </math> | ||
become [[linearly dependent]] and | become [[linearly dependent]] and there is no manifest choice of a physically meaningful linearly independent subset. | ||
The first-order (most important) energy including exchange is in almost all symmetry-adapted perturbation theories given by the following expression | In the late 1960s, the Eisenschitz-London approach was revived and different rigorous variants of ''symmetry adapted perturbation theory'' were developed. (The word symmetry refers here to the permutational symmetry of electrons, i.e., to the indistinguishability of electrons). The different approaches shared a major drawback: they were very difficult to apply in practice. Hence a somewhat less rigorous approach (''weak symmetry forcing'') was introduced: apply ordinary RS-PT (Rayleigh-Schrödinger perturbation theory) and introduce the intermolecular antisymmetrizer at appropriate places in the RS-PT equations. This approach leads to feasible equations, and, when electronically correlated monomer functions are used, weak symmetry forcing gives reliable results.<ref name = "Jeziorski"/><ref name = "Szalewicz"/> The first-order (most important) energy including exchange is in almost all symmetry-adapted perturbation theories given by the following expression | ||
:<math> | :<math> | ||
E^{(1)}_\mathrm{antisymmetric} = \frac{ \langle \Phi_0^A \Phi_0^B| V^{AB}\tilde{\mathcal{A}}^{AB}| \Phi_0^A \Phi_0^B \rangle} { \langle \Phi_0^A \Phi_0^B| \tilde{\mathcal{A}}^{AB}| \Phi_0^A \Phi_0^B \rangle} . | E^{(1)}_\mathrm{antisymmetric} = \frac{ \langle \Phi_0^A \Phi_0^B| V^{AB}\tilde{\mathcal{A}}^{AB}| \Phi_0^A \Phi_0^B \rangle} { \langle \Phi_0^A \Phi_0^B| \tilde{\mathcal{A}}^{AB}| \Phi_0^A \Phi_0^B \rangle} . | ||
</math> | </math> | ||
An early application of this equation was by [[Walter Heitler]] and [[Fritz London]] who gave the first quantum mechanical account of covalent bonding (in H<sub>2</sub>). They presented, and evaluated numerically, this formula; see [[Chemical_bond#Heitler-London_theory|chemical bond]]. | |||
In the case of chemical bonding (as in H<sub>2</sub>) first order interactions (with functions in bra and ket adapted to proper permutation symmetry) are attractive for certain electron-spin states, usually spin-singlet and responsible for large bonding energies—on the order of a hundred kcal/mol. In the case of intermolecular forces between closed shell systems, the same kind of interaction is strongly repulsive and responsible for the "volume" of the molecule (see [[Van der Waals radius]]). | |||
Roughly speaking, the exchange interaction is proportional to the differential overlap between Φ<sub>0</sub><sup>''A''</sup> and Φ<sub>0</sub><sup>''B''</sup>. Since the wave functions decay exponentially as a function of distance, the exchange interaction does too. Hence the range of action is relatively short, which is why exchange interactions are referred to as short range interactions. | Roughly speaking, the exchange interaction is proportional to the differential overlap between Φ<sub>0</sub><sup>''A''</sup> and Φ<sub>0</sub><sup>''B''</sup>. Since the wave functions decay exponentially as a function of distance, the exchange interaction does too. Hence the range of action is relatively short, which is why exchange interactions are referred to as short range interactions. | ||
==Electrostatic interactions== | ==Electrostatic interactions== | ||
| Line 94: | Line 98: | ||
</math> | </math> | ||
Within the [[Born-Oppenheimer approximation|clamped nuclei approximation]], the clamped nucleus α on ''A'' has position vector '''R'''<sub>α</sub> and its charge times the [[Dirac delta function]], ''Z''<sub>α</sub> δ('''r'''—'''R'''<sub>α</sub>), is the charge density of nucleus α. | |||
The total charge density ρ<sup>''A''</sup><sub>tot</sub> of monomer ''A'' is the sum of a nuclear contribution (sum of delta functions) and an electronic contribution (integral over electronic wave function squared): | |||
The total charge density of monomer ''A'' | |||
:<math> | :<math> | ||
\rho^A_\mathrm{tot}(\mathbf{r}) = \sum_{\alpha} Z_\alpha \delta(\mathbf{r}-\mathbf{R}_\alpha) - \rho^A_\mathrm{el}(\mathbf{r}) | \rho^A_\mathrm{tot}(\mathbf{r}) = \sum_{\alpha} Z_\alpha \delta(\mathbf{r}-\mathbf{R}_\alpha) - \rho^A_\mathrm{el}(\mathbf{r}) | ||
</math> | </math> | ||
with the electronic charge density given by an integral over ''n''<sub>''A''</sub> | with the electronic charge density given by an integral over ''n''<sub>''A''</sub> − 1 primed electron coordinates: | ||
:<math> | :<math> | ||
\rho^A_\mathrm{el}(\mathbf{r}) = n_A \int |\Phi^A_0(\mathbf{r}, \mathbf{r}'_2, \ldots, \mathbf{r}'_{n_A})|^2 d\mathbf{r}'_2 \cdots d\mathbf{r}'_{n_A}. | \rho^A_\mathrm{el}(\mathbf{r}) = n_A \int |\Phi^A_0(\mathbf{r}, \mathbf{r}'_2, \ldots, \mathbf{r}'_{n_A})|^2 d\mathbf{r}'_2 \cdots d\mathbf{r}'_{n_A}. | ||
| Line 111: | Line 114: | ||
which is nothing but the classical expression for the electrostatic interaction between two charge distributions. This shows that the first-order RS-PT energy is indeed equal to the electrostatic interaction between ''A'' and ''B''. | which is nothing but the classical expression for the electrostatic interaction between two charge distributions. This shows that the first-order RS-PT energy is indeed equal to the electrostatic interaction between ''A'' and ''B''. | ||
====Multipole expansion==== | ====Multipole expansion==== | ||
With present day computer soft- and hard-ware it is feasible to compute the electrostatic energy without any further approximations other than those applied in the computation of the monomer wave functions. In the past this was different and a further approximation was commonly introduced: ''V''<sup>''AB''</sup> was expanded in a (truncated) series in inverse powers of the intermolecular distance ''R''<sub>''AB''</sub>. This yields the ''multipole expansion'' of the electrostatic energy. Since its concepts still pervade the theory of intermolecular forces, it is presented here. | |||
We will use the following vector notation | |||
:<math> | |||
\mathbf{r} = (r,\, \widehat{\mathbf{r}}) \quad\hbox{with}\quad \widehat{\mathbf{r}} = (\theta,\, \phi), | |||
</math> | |||
where θ and φ are the [[spherical polar coordinates|spherical polar angles]] of '''r''' with respect to a coordinate system in the laboratory (a so-called space-fixed frame). The quantity | |||
<math>\scriptstyle \widehat{\mathbf{r}}</math> is in fact a unit vector along '''r''' and is defined by two polar angles. Further ''r'' is the length of '''r'''. | |||
In [[Multipole expansion (interaction)|this article]] the following expansion is proved | |||
:<math> | :<math> | ||
V^{AB} = \sum_{\ell_A=0}^\infty \sum_{\ell_B=0}^\infty (-1)^{\ell_B} \binom{2\ell_A+2\ell_B}{2\ell_A}^{1/2} | \begin{align} | ||
\sum_{M=-\ell_A-\ell_B}^{\ell_A+\ell_B} (-1)^{M} | V^{AB} &= \sum_{\ell_A=0}^\infty \sum_{\ell_B=0}^\infty (-1)^{\ell_B} \binom{2\ell_A+2\ell_B}{2\ell_A}^{1/2} \\ | ||
&\times \sum_{M=-\ell_A-\ell_B}^{\ell_A+\ell_B} (-1)^{M} R^{-(\ell_A+\ell_B +1)} | |||
C_{\ell_A+\ell_B,-M}(\widehat{\mathbf{R}}_{AB})\; \left[\mathbf{Q}^{\ell_A} \otimes \mathbf{Q}^{\ell_B} \right]^{\ell_A+\ell_B}_M | |||
\end{align} | |||
</math> | </math> | ||
with the [[Clebsch-Gordan coefficients|Clebsch-Gordan]] series defined by | with the [[Clebsch-Gordan coefficients|Clebsch-Gordan]] series defined by | ||
| Line 123: | Line 138: | ||
Q_{m_A}^{\ell_A} Q_{m_B}^{\ell_B}\;\langle \ell_A, m_A; \ell_B, m_B| \ell_A+\ell_B, M \rangle. | Q_{m_A}^{\ell_A} Q_{m_B}^{\ell_B}\;\langle \ell_A, m_A; \ell_B, m_B| \ell_A+\ell_B, M \rangle. | ||
</math> | </math> | ||
The [[Spherical_harmonics#Normalization|spherical harmonic]] in Racah normalization is defined by | |||
:<math> | :<math> | ||
C_{L,M}(\widehat{\mathbf{R}}_{AB}) \equiv \left[\frac{4\pi}{2L+1}\right]^{1/2}\; | |||
Y_{L,M}(\widehat{\mathbf{R}}_{AB}). | |||
</math> | </math> | ||
The function Y<sub>''L,M''</sub> is a normalized [[spherical harmonic]], while | The function Y<sub>''L,M''</sub> is a to unity normalized [[spherical harmonic]], while | ||
<math>Q^{\ell_A}_{m_A}</math> and <math>Q^{\ell_B}_{m_B}</math> are [[Spherical multipole moments#General spherical multipole moments|spherical multipole moment]] operators | <math>\scriptstyle Q^{\ell_A}_{m_A}</math> and <math>\scriptstyle Q^{\ell_B}_{m_B}</math> are [[Spherical multipole moments#General spherical multipole moments|spherical multipole moment]] operators | ||
:<math> | |||
Q^{\ell_A}_{m_A} \equiv \sum_{i=1}^{N_A} q_i r_i^{\ell_A} C_{\ell_A,m_A}(\widehat{\mathbf{r}}_i), | |||
</math> | |||
where the sum over ''i'' is over electrons and nuclei of monomer A; their charges are given by ''q''<sub> ''i''</sub>. An equivalent definition holds for the multipole operators of monomer B. | |||
Insertion of this expansion into the first-order (without exchange) expression gives a very similar expansion for the electrostatic energy, because the matrix element factorizes, | Insertion of this expansion into the first-order (without exchange) expression gives a very similar expansion for the electrostatic energy, because the matrix element factorizes, Thus, the ''multipole-expansion of the electrostatic interaction'' (an expansion in powers of 1/''R''<sub>''AB''</sub>) is, | ||
:<math> | :<math> | ||
\begin{align} | \begin{align} | ||
E^{(1)}_\mathrm{electrostatic} = & \sum_{\ell_A=0}^\infty \sum_{\ell_B=0}^\infty (-1)^{\ell_B} \binom{2\ell_A+2\ell_B}{2\ell_A}^{1/2} | E^{(1)}_\mathrm{electrostatic} = & \sum_{\ell_A=0}^\infty \sum_{\ell_B=0}^\infty (-1)^{\ell_B} \binom{2\ell_A+2\ell_B}{2\ell_A}^{1/2}\, R^{-(\ell_A+\ell_B+1)}_{AB} \\ | ||
&\sum_{M=-\ell_A-\ell_B}^{\ell_A+\ell_B} (-1)^{M} | &\times \sum_{M=-\ell_A-\ell_B}^{\ell_A+\ell_B} (-1)^{M} C_{\ell_A+\ell_B,-M}(\widehat{\mathbf{R}}_{AB})\; \left[\mathbf{M}^{\ell_A} \otimes \mathbf{M}^{\ell_B} \right]^{\ell_A+\ell_B}_M, | ||
\end{align} | \end{align} | ||
</math> | </math> | ||
with the ''permanent multipole moments'' defined by | with the ''permanent multipole moments'' defined by the [[expectation values]] | ||
:<math> | :<math> | ||
M^{\ell_A}_{m_A} \equiv \langle \Phi_0^A | Q^{\ell_A}_{m_A}| \Phi_0^A\rangle | M^{\ell_A}_{m_A} \equiv \langle \Phi_0^A | Q^{\ell_A}_{m_A}| \Phi_0^A\rangle | ||
| Line 144: | Line 163: | ||
M^{\ell_B}_{m_B} \equiv \langle \Phi_0^B | Q^{\ell_B}_{m_B}| \Phi_0^B\rangle . | M^{\ell_B}_{m_B} \equiv \langle \Phi_0^B | Q^{\ell_B}_{m_B}| \Phi_0^B\rangle . | ||
</math> | </math> | ||
The series is of infinite length, and, indeed, most molecules have an infinite number of non-vanishing multipoles. In the past, when computer calculations for the permanent moments were not yet feasible, it was common to truncate this series after the first non-vanishing term. | |||
It depends very much on the symmetry of the | It depends very much on the symmetry of the monomers which terms are non-vanishing,. For instance, molecules with an inversion center such as a homonuclear diatomic (e.g., [[Nitrogen#Molecular nitrogen (gas and liquid)|molecular nitrogen]] N<sub>2</sub>), or an organic molecule like [[ethene]] (C<sub>2</sub>H<sub>4</sub>) | ||
do not | do not possess a permanent dipole moment (''l'' = 1), but do carry a quadrupole moment (''l'' = 2). | ||
Molecules such a [[hydrogen chloride]] (HCl) and [[water]] (H<sub>2</sub>O) lack an inversion center and hence do have a permanent dipole. So, the first non-vanishing electrostatic term in, e.g., the N<sub>2</sub>—H<sub>2</sub>O dimer, is the ''l''<sub>''A''</sub> = 2, ''l''<sub>''B''</sub> = 1 term. From the formula above follows that this term | Molecules such a [[hydrogen chloride]] (HCl) and [[water]] (H<sub>2</sub>O) lack an inversion center and hence do have a permanent dipole. So, the first non-vanishing electrostatic term in, e.g., the N<sub>2</sub>—H<sub>2</sub>O dimer, is the ''l''<sub>''A''</sub> = 2, ''l''<sub>''B''</sub> = 1 term. From the formula above follows that this term is of order ''L'' = ''l''<sub>''A''</sub> + ''l''<sub>''B''</sub> = 3 and has an ''R''<sup>–4</sup> dependence. Note, parenthetically, that in this dimer the quadrupole-quadrupole interaction (''R''<sup>–5</sup>) is not unimportant either, because the water molecule carries a non-vanishing quadrupole as well. | ||
When computer calculations of permanent multipole moments of any order became possible, the matter of the convergence of the multipole series became urgent. | When computer calculations of permanent multipole moments of any order became possible, the matter of the convergence of the multipole series became more urgent. There are indications that for overlapping charge distributions the multipole expansion is divergent and is an [[asymptotic series]]. | ||
==== Ionic interactions ==== | ==== Ionic interactions ==== | ||
It is debatable whether ionic interactions are to be | It is debatable whether ionic interactions are to be seen as intermolecular forces, as some workers consider them rather as special kind of chemical bonding. | ||
The forces occur between charged atoms or molecules ([[ion]]s). | The forces occur between charged atoms or molecules ([[ion]]s). Ionic bonds are formed when the difference between the [[electron affinity]] of one monomer and the [[ionization potential]] of the other is so small in absolute value that electron transfer from the one monomer to the other | ||
is energetically favorable. Since a transfer of an electron is never complete there is always a degree of covalent bonding. | |||
Once the ions (of opposite sign) are formed, the interaction between them can seen as a special case of multipolar attraction, with a 1/''R''<sub>''AB''</sub> distance dependence. Indeed, the ionic interaction is the electrostatic term with ''l''<sub>''A''</sub> = 0 and ''l''<sub>''B''</sub> = 0. Using that the | Once the ions (of opposite sign) are formed, the interaction between them can be seen as a special case of multipolar attraction, with a 1/''R''<sub>''AB''</sub> distance dependence. Indeed, the ionic interaction is the electrostatic term with ''l''<sub>''A''</sub> = 0 and ''l''<sub>''B''</sub> = 0. Using that the ''L'' = 0 term is simply 1/''R''<sub>A''B''</sub> and that the monopole moments and their Clebsch-Gordan coupling are | ||
</ | |||
and that the monopole moments and their Clebsch-Gordan coupling are | |||
:<math> | :<math> | ||
M^{0_A}_0 = q_A,\quad M^{0_B}_0 = q_B \quad\hbox{and}\quad [\mathbf{M}^{0_A}\otimes \mathbf{M}^{0_A}]^0_0 = q_A q_B , | M^{0_A}_0 = q_A,\quad M^{0_B}_0 = q_B \quad\hbox{and}\quad [\mathbf{M}^{0_A}\otimes \mathbf{M}^{0_A}]^0_0 = q_A q_B , | ||
| Line 169: | Line 185: | ||
E^{(1)}_\mathrm{electrostatic} = \frac{q_A q_B}{R_{AB}} + \hbox{higher terms}. | E^{(1)}_\mathrm{electrostatic} = \frac{q_A q_B}{R_{AB}} + \hbox{higher terms}. | ||
</math> | </math> | ||
For shorter distances, where the charge distributions of the monomers overlap, the ions will repel each other because of intermonomer exchange of the electrons. | For shorter distances, where the charge distributions of the monomers overlap, the ions will repel | ||
each other because of intermonomer exchange of the electrons. | |||
Ionic compounds have high melting and boiling points due to the large amount of energy required to break the forces between the charged ions. When molten they are also good conductors of heat and electricity, due to the free or delocalised ions. | Ionic compounds have high melting and boiling points due to the large amount of energy required to break the forces between the charged ions. When molten they are also good conductors of heat and electricity, due to the free or delocalised ions. | ||
==== | =====Numerical example ===== | ||
Dipole-dipole interactions, also called | An example of a compound that is ionically bound is KCN. Due to the low ionization potential of potassium (4.339 eV) and the large electron affinity of CN (3.82 eV), the Coulomb attraction favors electron transfer from K to CN already at the large distance of 53 bohr (= 2.8 nm). For shorter distances the system can be regarded as to consist of two closed-shell monomers K<sup>+</sup> and CN<sup>−</sup> that show the kind of intermolecular interactions discussed in this article. It can be estimated from numbers given in Ref.<ref>P. E. S. Wormer and J. Tennyson, J. Chem. Phys. vol '''75''', pp. 1245-1253 (1981)</ref> that the | ||
dissociation energy of KCN → K + CN is about 75 kcal/mol (314 kJ/mol), which is the order of magnitude of a chemical binding energy. However, the motion of K<sup>+</sup> around CN<sup>−</sup> is without appreciable energy barrier, whereas a covalent bond is characterized by a strong directionality. In that sense the K<sup>+</sup> — CN<sup>−</sup> molecule indeed could be regarded as bound by intermolecular forces. | |||
==== Dipole-dipole interactions ==== | |||
Dipole-dipole interactions, also called Keesom forces after [[Willem Hendrik Keesom]], who gave the first mathematical description in 1921<ref name = "Keesom">W. H. Keesom, Phys. Zeitschrift, vol. '''22''' p. 129 (1921)</ref>, are the forces that occur between two molecules with [[molecular dipole|permanent dipoles]]. An example of this can be seen in [[hydrochloric acid]]: | |||
[[Image:HCl_dimer_dipole.png|center|300px]] | [[Image:HCl_dimer_dipole.png|center|300px]] | ||
The molecules are depicted here as two point | The molecules are depicted here as two [[electric dipole|point dipole]]s in the energetically most unfavorable position. A point dipole is an idealization similar to a point charge (a finite charge in an infinitesimally small volume). A point dipole consists of | ||
two equal charges of opposite sign δ<sup>+</sup> and δ<sup>–</sup> that are a distance ''d'' apart. The distance ''d'' is so small that at any distance ''R'' away from the point dipole it | two equal charges of opposite sign δ<sup>+</sup> and δ<sup>–</sup> that are a distance ''d'' apart. The distance ''d'' is so small that at any distance ''R'' away from the point dipole, it holds that ''d/R'' >> (''d/R'')<sup>2</sup>. In this limit the electrostatic field outside the charge distribution consists of one (''R''<sup>−3</sup>) term only, see [[electric dipole]]. The electrostatic interaction between two point dipoles is given by the single term ''l''<sub>''A''</sub> = 1 and ''l''<sub>''B''</sub> = 1 in the expansion above. | ||
Obviously, no molecule is an ideal point dipole, and in the case of the HCl dimer, for instance, dipole-quadrupole, quadrupole-quadrupole, etc. interactions are by no means negligible (and neither | Obviously, no molecule is an ideal point dipole, and in the case of the HCl dimer, for instance, dipole-quadrupole, quadrupole-quadrupole, etc., interactions are by no means negligible (and neither induction nor dispersion interactions can be neglected). | ||
Note that | Note that the dipole-dipole interaction involving a closed-shell atom is zero, because a closed-shell atom does not carry a permanent dipole. | ||
In order to obtain the mathematical equation for the dipole-dipole interaction, we must consider in the expansion of the electrostatic energy the term with ''l''<sub>''A''</sub> = 1 and ''l''<sub>''B''</sub> = 1. Because this expansion is termwise rotationally invariant, we can choose a convenient system of axes to evaluate the term. We choose a coordinate system centered on ''A'' with its ''z''-axis coinciding with the intermolecular vector '''R'''<sub>''AB''</sub>. | |||
Under this circumstance it holds for | Under this circumstance it holds for Racah spherical harmonic that | ||
:<math> | :<math> | ||
C_{\ell m}(\mathbf{R}_{AB}) \equiv \sqrt{\frac{4\pi}{2\ell+1}} \; Y_{\ell m}(0,0) = \delta_{0m} . | |||
</math> | </math> | ||
Hence, the dipole-dipole term becomes after substitution of two [[Clebsch-Gordan coefficients]] | Hence, the dipole-dipole term becomes after substitution of two [[Clebsch-Gordan coefficients]] | ||
| Line 204: | Line 225: | ||
E_{\mathrm{dip-dip}} = \frac{1}{R^{3}_{AB}}\left[ \mu_x^A\mu_x^B + \mu_y^A\mu_y^B - 2\mu_z^A\mu_z^B \right]. | E_{\mathrm{dip-dip}} = \frac{1}{R^{3}_{AB}}\left[ \mu_x^A\mu_x^B + \mu_y^A\mu_y^B - 2\mu_z^A\mu_z^B \right]. | ||
</math> | </math> | ||
Introducing the [[dot product]], we may write | |||
:<math> | :<math> | ||
\boldsymbol{\mu}^A = (\mu_x^A, \mu_y^A, \mu_z^A) \quad\hbox{and}\quad | \boldsymbol{\mu}^A = (\mu_x^A, \mu_y^A, \mu_z^A) \quad\hbox{and}\quad | ||
| Line 214: | Line 235: | ||
</math> | </math> | ||
As a numerical example we consider the HCl dimer depicted above. | =====Numerical example===== | ||
As a numerical example we consider the HCl dimer depicted above. The left molecule is called ''A'' and the right ''B'', so that the ''z''-axis is along the molecules and points to the right. ''The present convention of the dipole vector is such that it points from negative to positive charge''. <ref>This convention of direction of dipole, while common in physics, is opposite to the one used in organic chemistry. Since organic chemists hardly ever perform vector computations with dipoles, confusion hardly ever arises. In organic chemistry dipoles are mainly used in semi-quantitative discussions of charge separation in a molecule.</ref> | |||
Hence (for clarity reasons we change the monomer label from superscript to subscript), | |||
:<math> | :<math> | ||
\boldsymbol{\mu} | \boldsymbol{\mu}_A = \mu_\mathrm{HCl} | ||
\begin{pmatrix} 0 \\ 0 \\ 1 \end{pmatrix} | \begin{pmatrix} 0 \\ 0 \\ 1 \end{pmatrix} | ||
\quad\hbox{and}\quad\boldsymbol{\mu} | \quad\hbox{and}\quad\boldsymbol{\mu}_B = \mu_\mathrm{HCl} | ||
\begin{pmatrix} 0 \\ 0 \\ -1 \end{pmatrix} | \begin{pmatrix} 0 \\ 0 \\ -1 \end{pmatrix} | ||
</math> | </math> | ||
| Line 226: | Line 248: | ||
E_{\mathrm{dip-dip}} = \frac{2\mu^2_\mathrm{HCl}}{R^{3}_{AB}}. | E_{\mathrm{dip-dip}} = \frac{2\mu^2_\mathrm{HCl}}{R^{3}_{AB}}. | ||
</math> | </math> | ||
The value of μ<sub>HCl</sub> is 0.43 ([[atomic units]]), so that at a distance of 10 [[bohr radius|bohr]] the dipole-dipole repulsion is 3.698 10<sup>–4</sup> [[hartree energy|hartree]] (0.97 kJ/mol). If we rotate molecule B over 180<sup>0</sup> degree, the negative chlorine atom comes close to the positive hydrogen atom of the other monomer and there is an attraction of 0.97 kJ/mol. | The value of μ<sub>HCl</sub> is 0.43 ([[atomic units]]), so that at a distance of 10 [[bohr radius|bohr]] the dipole-dipole repulsion is 3.698 10<sup>–4</sup> [[hartree energy|hartree]] (0.97 kJ/mol). If we rotate molecule B over 180<sup>0</sup> degree, the negative chlorine atom comes close to the positive hydrogen atom of the other monomer and there is an attraction of 0.97 kJ/mol. | ||
If one of the molecules is neutral and freely rotating, the total electrostatic interaction energy becomes zero, since all terms in the multipole expansion vanish upon free rotation, except the monopole-monopole interaction. (For the dipole-dipole interaction this is most easily proved by integrating over the spherical polar angles of the dipole vector, while using the volume element sinθ dθdφ). In gases and liquids the molecules are not rotating freely: the rotation is weighted by the [[Boltzmann factor]] exp( | =====Thermal rotation===== | ||
It was first shown by | If one of the molecules is neutral and freely rotating, the total electrostatic interaction energy becomes zero, since all terms in the multipole expansion vanish upon free rotation, except the monopole-monopole interaction. (For the dipole-dipole interaction this is most easily proved by integrating over the [[spherical polar coordinates|spherical polar angles]] of the dipole vector, while using the volume element sinθ dθdφ). In gases and liquids the molecules are not rotating freely: for dipolar molecules the rotation is weighted by the [[Boltzmann factor]] exp(−''E''<sub>dip-dip</sub>/''kT''), where ''k'' is the [[Boltzmann constant]] and ''T'' the absolute temperature. | ||
It was first shown by W.H. Keesom <ref name = "Keesom" /> that the temperature-averaged dipole-dipole interaction is | |||
:<math> | :<math> | ||
\overline{E}_\mathrm{dip-dip} = -\frac{2 | \overline{E}_\mathrm{dip-dip} = -\frac{2 \mu_A^2\,\mu_B^2}{3R_{AB}^6 kT}. | ||
</math> | </math> | ||
Since the averaged energy has an R<sup>& | Since the thermally averaged energy has an ''R''<sup>−6</sup> dependence, it is evidently much weaker than the unaveraged energy that has an ''R''<sup>−3</sup> dependence, but it is not completely zero. It is attractive, because the Boltzmann weighting favors somewhat the attractive regions of space. In HCl–HCl we find for T = 300 K and ''R''<sub>''AB''</sub> = 10 bohr (0.53 nm) the averaged attraction −62 J/mol, which shows a weakening, due to thermal rotational motion, of the interaction by a factor of about 16. In the literature there is much confusion about the size of the dipole-dipole interaction, because some workers quote unaveraged values, while others quote thermally averaged values—the two differ by more than an order of magnitude. | ||
==== Hydrogen bonding ==== | ==== Hydrogen bonding ==== | ||
{{main|Hydrogen bond}} | {{main|Hydrogen bond}} | ||
[[Hydrogen bonding]] is an intermolecular interaction with a [[hydrogen | [[Hydrogen bonding]] is an intermolecular interaction with a [[hydrogen]] atom being present in the intermolecular bond. This hydrogen is covalently (chemically) bound in one molecule, which acts as the ''proton donor.'' The other molecule acts as the ''proton acceptor''. | ||
In the following important example of the water dimer, the [[ | In the following important example of the water dimer, the [[water]] molecule on the right is the proton donor, while the one on the left is the proton acceptor: | ||
[[Image:H2O_dimer.png|center|250px]] | [[Image:H2O_dimer.png|center|250px]] | ||
The | The donated proton in a hydrogen bond is usually bound to an [[electronegativity|electronegative]] atom of the donor molecule. Examples of electronegative atoms are [[nitrogen]], [[oxygen]], and [[fluorine]]. The electronegative atom is negatively charged (carries a charge δ<sup>-</sup>) and the hydrogen atom bound to it is positively charged. Consequently the proton donor is a polar molecule with a relatively large dipole moment. Often the positively charged hydrogen atom points towards an electron rich region in the acceptor molecule. The fact that an electron rich region exists in the acceptor molecule, implies already that the acceptor has a relatively large dipole moment as well. The result is a dimer that to a large extent is bound by the dipole-dipole force. | ||
For quite some time it was believed that hydrogen bonding required an explanation that was different from the other intermolecular interactions. However, reliable computer calculations that became possible since the 1980s, have shown that only the four effects listed above play a role, with the dipole-dipole interaction being singularly important. Since the four effects account completely for the bonding in small dimers like the water dimer, for which highly accurate calculations are feasible, it is now generally believed that no other bonding effects are operative. | For quite some time it was believed that hydrogen bonding required an explanation that was different from the other intermolecular interactions. However, reliable computer calculations that became possible since the 1980s, have shown that only the four effects listed above play a role, with the dipole-dipole interaction being singularly important. Since the four effects account completely for the bonding in small dimers like the water dimer, for which highly accurate calculations are feasible, it is now generally believed that no other bonding effects are operative. | ||
Hydrogen bonds are found throughout nature. They give water its unique properties that are so important to life on earth. Hydrogen bonds between hydrogen atoms and nitrogen atoms of adjacent base pairs provide the intermolecular force that bind together the two strands in a molecule of [[DNA]]. | Hydrogen bonds are found throughout nature. They give water its unique properties that are so important to life on earth. Hydrogen bonds between hydrogen atoms and nitrogen atoms of adjacent base pairs provide the intermolecular force that bind together the two strands in a molecule of [[DNA]]. | ||
To give an indication of the order of magnitude of a typical hydrogen-bonding energy, we quote the estimate ''D''<sub>e</sub> = −5.00 kcal/mol = −20.9 kJ/mol for the equilibrium energy of the water dimer.<ref>E. M. Mass, ''et al.'', J. Chem. Phys. vol '''113''', pp. 6687-6701 (2000).</ref> | |||
==Induction (polarization) forces== | ==Induction (polarization) forces== | ||
| Line 270: | Line 295: | ||
In the first term on the right hand side ''A'' is in its ground state and polarizes ''B'', in the second term ''B'' is in its ground state and polarizes ''A''. The third term gives dispersion interaction and will be discussed in a later section. | In the first term on the right hand side ''A'' is in its ground state and polarizes ''B'', in the second term ''B'' is in its ground state and polarizes ''A''. The third term gives dispersion interaction and will be discussed in a later section. | ||
It is possible to give general, non-multipole-expanded, expressions for the induction (without exchange). To this end we need to introduce a polarization propagator and to give an expression for the electric field generated by a molecule. [See, for instance, Eq. (19) in Ref.<ref name = "Jeziorski" />]. It is also possible to use the multipole expansion and by an angular momentum recoupling procedure<ref name "Wormer">P. E. S. Wormer, F. Mulder, and A. van der Avoird, ''Quantum Theoretical Calculations of Van der Waals Interaction between Molecules. Anisotropic Long Range Interactions'', International Journal of Quantum Chemistry, vol. '''11''', pp. 959-970 (1977)</ref> to express the polarization energy in terms of monomer properties. Both approaches are outside the scope of this article. A simple formula is obtained if both monomers are assumed to rotate freely. In contrast to the electrostatic energy, the rotationally averaged induction interaction is not zero, due to the definiteness of the second order RS-PT energy. | It is possible to give general, non-multipole-expanded, expressions for the induction (without exchange). To this end we need to introduce a polarization propagator and to give an expression for the electric field generated by a molecule. [See, for instance, Eq. (19) in Ref.<ref name = "Jeziorski" />]. It is also possible to use the multipole expansion and by an angular momentum recoupling procedure<ref name = "Wormer">P. E. S. Wormer, F. Mulder, and A. van der Avoird, ''Quantum Theoretical Calculations of Van der Waals Interaction between Molecules. Anisotropic Long Range Interactions'', International Journal of Quantum Chemistry, vol. '''11''', pp. 959-970 (1977)</ref> to express the polarization energy in terms of monomer properties. Both approaches are outside the scope of this article. | ||
We define | |||
A simple formula is obtained if both monomers are assumed to rotate freely. In contrast to the electrostatic energy, the rotationally averaged induction interaction is not zero, due to the negative definiteness of the second order RS-PT energy. | |||
We define a rotationally invariant and positive property, which is proportional to the squared length of the multipole moment, | |||
:<math> | :<math> | ||
|\mathbf{M}^{\ell_A}|^2 \equiv | |||
\frac{1}{2\ell_A+1} \sum_{m=-\ell_A}^{\ell_A} \langle \Phi_0^A | (Q^{\ell_A}_m )^* |\Phi_0^A\rangle | \frac{1}{2\ell_A+1} \sum_{m=-\ell_A}^{\ell_A} \langle \Phi_0^A | (Q^{\ell_A}_m )^* |\Phi_0^A\rangle | ||
\langle \Phi_0^A | Q^{\ell_A}_m |\Phi_0^A\rangle | \langle \Phi_0^A | Q^{\ell_A}_m |\Phi_0^A\rangle | ||
| Line 287: | Line 314: | ||
:<math> | :<math> | ||
\overline{E}_{\mathrm{ind}} = \sum_{\ell_A, \ell_B}\;\binom{2\ell_A+2\ell_B}{2\ell_A}\; | \overline{E}_{\mathrm{ind}} = \sum_{\ell_A, \ell_B}\;\binom{2\ell_A+2\ell_B}{2\ell_A}\; | ||
\frac{ | \frac{|\mathbf{M}^{\ell_A}|^2\, A^{\ell_B}_0}{ R^{2\ell_A+2\ell_B+2}}\; + \;\hbox{A and B interchanged} . | ||
</math> | |||
The most important term for neutral subsystems is for ''l''<sub>A</sub> = ''l''<sub>B</sub> = 1. The usual dipole [[polarizability]] α<sub>0</sub> is proportional to the generalized isotropic polarizability and |'''M'''<sup>1</sup>|<sup>2</sup> is proportional to the square of the length of the dipole '''μ''': | |||
:<math> | |||
A^{1}_0 = - \frac{1}{2} \alpha_0 \quad\hbox{and} \quad |\mathbf{M}^{1}|^2 = \frac{1}{3} \boldsymbol{\mu}\cdot\boldsymbol{\mu} = \frac{1}{3} \mu^2, | |||
</math> | </math> | ||
where the [[dot product]] of '''μ''' with itself is introduced. | |||
The isotropic polarizability α<sub>0</sub> is defined by | |||
:<math> | :<math> | ||
\alpha_0 \equiv \frac{1}{3} \sum_{i=x,y,z} \alpha_{ii} \quad\hbox{and}\quad | |||
\alpha_{ii} \equiv | |||
2 \sum_{k>0}^{\infty} | |||
\frac{ \langle \Phi_0 | \mu_i |\Phi_k\rangle | |||
\langle \Phi_k | \mu_i |\Phi_0\rangle} {E_k-E_0}. | |||
</math> | </math> | ||
Hence the first induction terms are | Hence the first induction terms are | ||
| Line 298: | Line 334: | ||
E_{A \leftarrow B } = - \frac{\alpha_0^A (\mu^B)^2}{ R^{6}}. | E_{A \leftarrow B } = - \frac{\alpha_0^A (\mu^B)^2}{ R^{6}}. | ||
</math> | </math> | ||
As a numeric example we consider HCl again. Its polarizability α<sub>0</sub> is 17.5 [[atomic units|au]]. Polarization of one monomer by the dipole of the other at ''R'' = 10 | As a numeric example we consider HCl again. Its polarizability α<sub>0</sub> is 17.5 [[atomic units|au]]. Polarization of one monomer by the dipole of the other at ''R'' = 10 bohr gives an attraction of 3.2 10<sup>−6</sup> hartree, which corresponds to −8.5 J/mol. | ||
Twice this number (−17 J/mol) may be compared with the thermally averaged electrostatic energy −62 J/mol mentioned above. | Twice this number (−17 J/mol) may be compared with the thermally averaged electrostatic energy −62 J/mol mentioned above. | ||
== London (dispersion) forces == | |||
In 1908 [[Kamerling Onnes]] and his coworkers liquified helium, which experimentally proved that two helium atoms attract each other—so that a liquid can be formed—but also that they repel each other—so that the liquid does not implode. Earlier (1873) [[Johannes Diderik van der Waals|Van der Waals]] had already assumed on theoretical grounds that there must be attraction and repulsion between atoms. He made this assumption in the derivation of his [[van der Waals equation|equation of state]]. | |||
'''( | Until the advent of quantum mechanics it was an enigma why two noble gas atoms would repel or attract each other. Shortly after the introduction of the [[Schrödinger equation]], Wang (1927) | ||
solved this equation perturbatively for two ground-state hydrogen atoms at large interatomic distance ''R''. Approximating the electronic interaction by a Taylor series in ''1/R'' he found an attractive potential with as leading term −''C''<sub>6</sub>/''R''<sup>6</sup>. | |||
A few years later (1930) [[London, United Kingdom|London]]<ref name="London">F. London, Zeitschrift für Physik, vol. '''63''', pp. 245-279 (1930) [http://dx.doi.org/10.1007/BF01421741 doi] and Z. Physik. Chemie, vol. B'''11''', p. 222 (1930). English translations in H. Hettema, ''Quantum Chemistry, Classic Scientific Papers,'' World Scientific, Singapore (2000).</ref> showed that so-called [[oscillator strengths]] appear in the equations for the intermolecular interactions. Oscillator strengths were known from the classical theory of the [[dispersion of light]] developed by [[Drude]] and [[Lorentz]]. Also later, in the quantum mechanical dispersion theory of [[Kramers]] and [[Heisenberg]], these quantities played a central role. Because of the similarity of his perturbation theory with the older dispersion theory, London coined the name ''dispersion effect'' for the attraction between noble gas atoms. It is common today to refer to these attractive long-range forces as ''London'' or ''dispersion forces''. | |||
The dispersion force acts between any two molecules and is clearly the only long-range interaction between two noble gas atoms, as noble gases do not have non-vanishing multipoles. No long range electrostatic or induction interactions are operative between noble gas atoms. After London's quantum mechanical account of the dispersion attraction, many workers have tried to find a classical explanation. So far this has not led to any quantitative mathematical expressions, but only to handwaving arguments in which ''instantaneous dipoles'' (vectors of undetermined direction and magnitude) play an important role. | |||
The dimers that interact mainly through dispersion forces are usually very weakly bound. In the CO-dimer the equilibrium energy ''D''<sub>e</sub> is about 1.5 kJ/mol, but this value is not due to dispersion alone. Recall that the carbon monoxide molecule has a small dipole and a reasonable quadrupole, which contribute to the interaction as well. | |||
For noble gas pairs, dispersion is the only attracting force. For instance, He<sub>2</sub> has the equilibrium energy ''D''<sub>e</sub> = 0.091 kJ/mol. The dimer Ar<sub>2</sub> has an equilibrium energy of 1.1 kJ/mol, which is more than an order of magnitude larger than the value of the helium dimer. As we will see below, this is due to the fact that the polarizability of the argon atom (11.09 bohr<sup>3</sup>) is much larger than that of the helium atom (1.38 bohr<sup>3</sup>). A large factor (∼ 48.2) appears also in the ratio of the ''C''<sub>6</sub> values of Ar<sub>2</sub> and He<sub>2</sub>, which are 70.8 and 1.47 au, respectively. The ratio between the equilibrium energy of Ar<sub>2</sub> and He<sub>2</sub> (∼ 12.1) is less than would be expected from the ratio between their ''C''<sub>6</sub> values. This is because the equilibrium distance in He<sub>2</sub> (5.6 bohr) is less than that in Ar<sub>2</sub> (7.5 bohr), so that the ''C''<sub>6</sub>/''R''<sup>6</sup> term in the case of the helium dimer has a factor (7.5/5.6)<sup>6</sup> = 5.8 more weight than in Ar<sub>2</sub>. | |||
=== Quantum mechanical theory of dispersion forces=== | |||
Above the quantum mechanical second-order RS-PT expression is given: | |||
:<math> | |||
E_\mathrm{disp} \equiv \sum_{n>0}^\infty \sum_{m>0}^\infty | |||
\frac{|\langle \Phi^A_0 \Phi^B_0 |V^{AB}|\Phi^A_n \Phi^B_m \rangle|^2} | |||
{E_0^A + E_0^B - E_n^A - E_m^B}. | |||
</math> | |||
London developed the perturbation ''V''<sup>''AB''</sup> in a series in ''R''<sup>−1</sup>, where ''R'' is the distance between the nuclear [[centers of mass]] of the monomers. This series is the [[Intermolecular forces#Multipole expansion|multipole expansion]] given above. When substituted into the second-order expression, ''E''<sub>disp</sub> is also obtained as series in ''R''<sup>−1</sup>, with the first term being −''C''<sub>6</sub>/''R''<sup>6</sup>. | |||
'' | Much later (in the early 1980s) it was shown by several workers independently that the multipole expansion is not needed. It is possible to reformulate ''E''<sub>disp</sub> in terms of ''frequency-dependent polarization propagators''. This work was a generalization of earlier work by Casimir and Polder (1948), who—using the multipole expansion—showed that the first term in the 1/''R'' expansion can be written as the following integral | ||
:<math> | |||
C_6 = \frac{3}{\pi} \int_0^{\infty} \alpha^{A}(i\omega) \alpha^{B}(i\omega) | |||
d\omega. | |||
</math> | |||
Here α<sup>''A''</sup>(iω) and α<sup>''B''</sup>(iω) are the frequency-dependent dipole polarizabilities of the respective atoms. The frequency ω is multiplied by the imaginary unit ''i''. It is not difficult to give Casimir-Polder type expressions for the other terms in the expansion. In these higher terms one meets quadrupole, octupole, ... and mixed-pole (for example dipole-quadrupole) polarizabilities. | |||
London obviously did not know the Casimir-Polder integral in 1930 and he made an additional approximation, named after [[Albrecht Unsöld]]. In this approximation one assumes that the excitation energies in the denominator of the second-order energies can be moved in front of the sum. In the summand remains an expression which is (almost) the unit operator—in its form of a resolution in terms of basis elements of Hilbert space (see [[closure relation]]). | |||
In this manner London obtained dispersion in terms of static (ω = 0) dipole polarizabilities and [[ionization potential]]s. He found the following approximation for the dispersion interaction ''E''<sub>disp</sub> between two atoms ''A'' and ''B''. The quantities ''I''<sub>''A''</sub> and ''I''<sub>''B''</sub> are the first ionization potentials of the atoms, | |||
:<math> | |||
E_\mathrm{disp} \approx | |||
-{3 \alpha^A(0) \alpha^B(0) I_A I_B\over 2(I_A + I_B)} R^{-6} . | |||
-{3 \alpha^A \alpha^B I_A I_B\over 2(I_A + I_B)} R^{-6} | |||
</math> | </math> | ||
Application of this London formula to the dispersion interaction between two hydrogen atoms [α(0) = 4 au, ''I'' = 0.5 hartree] gives ''C''<sub>6</sub> = 6 au. The accurate value is 6.499 au. | |||
It was pointed out by Hirschfelder ''et al.'' in their authoritative treatise on intermolecular forces,<ref>J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, Wiley, New York, (1954); page 955.</ref> that no instantaneous dipoles are apparent in the present quantum mechanical formulation of dispersion. In the article [[dispersion interaction]] it is shown that an equation for ''E'', of a similar form as the one above, appears in a time-independent quantum mechanical treatment of two oscillating dipoles that are interacting electrostatically. | |||
Qualitatively the magnitude of a polarizability is a measure for the size of an atom or molecule. | |||
This is clearly demonstrated by the following dipole polarizabilities of the noble gases (in bohr<sup>3</sup>) from<ref name="Standard">J. M. Standard and P. R. Certain, J. Chemical Physics, vol. '''83''', pp. 3002 - 3008 (1985)</ref> | |||
:: He 1.383 | |||
:: Ne 2.668 | |||
:: Ar 11.09 | |||
:: Kr 16.74 | |||
:: Xe 27.0 | |||
Since the dispersion ''C''<sub>6</sub> values are proportional to dipole polarizabilities, | |||
the London dispersion forces are proportional to the sizes of the monomers as is shown by the following reasonably accurate upper bounds from Ref.<ref name="Standard" /> (''C''<sub>6</sub> values in atomic units): | |||
::He-He 1.47 Ne-Xe 46.5 | |||
::He-Ne 3.20 Ar-Ar 70.8 | |||
::He-Ar 10.1 Ar-Kr 100. | |||
::He-Kr 14.2 Ar-Xe 151 | |||
::He-Xe 21.4 Kr-Kr 142 | |||
::Ne-Ne 7.27 Kr-Xe 215 | |||
::Ne-Ar 22.0 Xe-Xe 325 | |||
::Ne-Kr 30.9 | |||
== Anisotropy and non-additivity of intermolecular forces == | == Anisotropy and non-additivity of intermolecular forces == | ||
Consider the interaction between two electric point charges at position | Consider the interaction between two electric point charges at position | ||
<math>\vec{r}_1</math> and <math>\vec{r}_2</math>. By | <math>\vec{r}_1</math> and <math>\vec{r}_2</math>. By | ||
[[ | [[Coulomb's law]] the interaction potential | ||
depends only on the distance <math>|\vec{r}_1-\vec{r}_2|</math> between | depends only on the distance <math>|\vec{r}_1-\vec{r}_2|</math> between | ||
the particles. For molecules this is different. If we see a molecule as | the particles. For molecules this is different. If we see a molecule as | ||
| Line 375: | Line 427: | ||
:<math> U = u(r_{12})+ u(r_{13})+u(r_{23}) +u(r_{12},r_{13},r_{23}),</math> | :<math> U = u(r_{12})+ u(r_{13})+u(r_{23}) +u(r_{12},r_{13},r_{23}),</math> | ||
where <math> u( r_{12},r_{13},r_{23}) </math> is a ''non-additive three-body'' interaction. | where <math> u( r_{12},r_{13},r_{23}) </math> is a ''non-additive three-body'' interaction. | ||
Such an interaction can be caused by | Such an interaction can be caused by<ref> R. Moszynski, P. E. S. Wormer, B. Jeziorski, and A. van der Avoird, ''Symmetry-Adapted Perturbation Theory of Nonadditive Three-Body Interactions in van der Waals Molecules. I. General Theory'', J. Chem. Phys. vol. '''103''', pp. 8058-8074 (1995)</ref> exchange interactions, by induction, and by dispersion (the latter is known as the Axilrod-Teller triple dipole effect). | ||
dispersion (the | |||
==References== | ==References== | ||
{{reflist|2}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:01, 1 September 2024
- See Van der Waals forces for a brief overview.
In physics, chemistry, and biology, intermolecular forces are forces that act between stable molecules or between functional groups of macromolecules. Sometimes they are referred to as Van der Waals forces, although some texts apply the latter term to a specific subset of intermolecular forces.
Intermolecular forces are non-covalent and give rise to bonding energies of a few kcal/mol or less, i.e., they are generally much weaker than chemical bonding (covalent) forces. Nevertheless, intermolecular forces are the cause of a wide range of physical, chemical, and biological phenomena. For instance, they are responsible for the deviation from the ideal gas law for real gases, determine the tertiary structure of macromolecules, and explain why some relatively light molecules are liquid or solid at normal temperature and pressure.
In general one distinguishes short- and long-range intermolecular forces. As will be explained in this article, the former are mainly due to the intermolecular exchange of electrons, a phenomenon closely related to the quantum mechanical Pauli principle. Short-range forces fall off exponentially as a function of intermolecular distance R and are repulsive for interacting stable (closed-shell) moieties. In chemistry they are well known, because they give rise to steric hindrance, also known as Born or Pauli repulsion.
The long-range forces, on the other hand, fall off with inverse powers of the distance, R −n, typically 3 ≤ n ≤ 10, and are mostly attractive. They are partly caused by interaction between permanent molecular dipoles and higher multipoles, by induction effects, and by a quantum mechanical effect for which Fritz London coined the name dispersion effect.
The sum of the attractive long-range and repulsive short-range forces gives rise to a minimum, referred to as Van der Waals minimum. The position and depth of the Van der Waals minimum depends on distance and mutual orientation of the molecules.
Observation versus computation
In general, a force is minus the gradient (set of derivatives with respect to coordinates) of a potential. Therefore, it is common to concentrate on the intermolecular potential rather than on its derivatives—the intermolecular force. An intermolecular potential—a scalar function of the positions of the nuclei constituting a molecular complex—cannot be measured directly. Experimental information is obtained from measurable properties that depend on the intermolecular potential. The standard example is the pressure-volume-temperature (P-V-T) dependence of a van der Waals fluid. After measurement of the P-V-T relation, the two parameters in the van der Waals equation can be determined and these parameters tell something about the interaction in a pair of molecules constituting the fluid. Clearly, by a simple extension the same holds for more elaborate equations of state, i.e., for multiparameter P-V-T relations.
Other examples of situations where the intermolecular potential plays a role, are transport properties of fluids, molecular collision cross-sections, spectra of molecular complexes (dimers, trimers, …), and structures of molecular solids. In all these cases the observed properties depend on the positions of the nuclei via the intermolecular potential. The dependence of the property on the potential can be simple, as in the example of a van der Waals gas, or through a set of elaborate classical or quantum mechanical equations. However, all these observable properties have in common that the road backward from experimental values to the intermolecular potential is non-unique. Very different potentials may predict the same observables. Before the advent of computers, it was common to assume a simple model potential, containing a few adjustable parameters, and to modify these parameters so that an optimum match with the observations was found. Almost always these potentials lacked transferability, the potential applied to other properties of the same molecular complex fails in predicting properties other than those considered in the matching procedure.
In the last decade of the 20th century it became possible to compute the interaction energy of two (fairly small) molecules ab initio (from the beginning), that is, by solving the Schrödinger equation of the interacting molecules without any empirical input other than nuclear charges. Of course, many approximations have to be made along the way, but all of these are well investigated and their effects are known quantitatively. At present, therefore, the most reliable source of quantitative knowledge of intermolecular potentials is by ab initio computation. Once the potential has been calculated, one can go to the observable properties. This second step involves the solution of the equation of motion of the nuclei with the computed potential as input. The equation may be classical, associated with the names of Newton, Lagrange, or Hamilton, or the equation may be the quantum mechanical Schrödinger equation. The choice depends on whether or not quantum effects are important for the property under consideration.
The present article describes the most important effects that contribute to the intermolecular potential and that must be included in the ab initio computation of the potential.
Historical background and the need for quantum mechanics
Before the advent of quantum mechanics (middle of the 1920s), the origin of intermolecular forces was unknown.[1] Especially the causes of hard sphere repulsion between molecules, postulated by Van der Waals, and the possibility of the liquefaction of noble gases, first observed (1908) by Heike Kamerlingh Onnes for helium, were very puzzling, as these effects could not be explained at all by classical electromagnetic theory or by the "old" quantum theory. Soon after the formulation of the "new" quantum mechanics the pieces of the puzzle fell together and all open questions regarding intermolecular forces were answered, first by S.C. Wang and then more completely and thoroughly by Fritz London.
One of the more important effects is the dispersion interaction, a term coined in 1930 by London. This attractive interaction is present for all atom- and molecule-pairs, even for noble gas atoms. In fact, the dispersion force explains why helium gas can be liquefied. The dispersion force is often explained as due to an interaction between instantaneous electric dipoles. The term "instantaneous" suggests time dependence and some textbooks add that the instantaneous dipole-dipole interaction can be described classically, i.e., without quantum mechanics. London (also in 1930) gave a model of two oscillating electric dipoles interacting through an electrostatic Coulomb force and showed that time-independent quantum mechanics describes this attraction. Moreover, London emphasized that the zero-point motion of the oscillators, a typical quantum mechanical effect related to Heisenberg's uncertainty relation, is a necessary part of the explanation. Neither classical theory, nor the "old" Planck-Bohr quantum theory accounts for zero-point motion. In the article dispersion interaction London's oscillating dipole model is discussed in more detail. The present article will sketch the theory of dispersion, repulsion, and other intermolecular forces and by necessity will invoke a few quantum mechanical concepts.
Effects like dipole-dipole interaction and dipole-induced dipole interaction can be accounted for by classical theories provided the values of molecular dipoles and molecular polarizabilities are known. However, classical theory cannot explain why a molecule is a stable charge distribution, i.e., why the negative electrons do not fall down on the positive nuclei while losing their energy by emitting electromagnetic radiation. This also means that classical theory cannot predict the size of a molecular dipole or of a molecular polarizability. Quantum mechanics, on the other hand, does not only account qualitatively for all observed effects, but also quantitatively. As pointed out above, today's computer calculations, based on quantum physics, form a reliable source of quantitative information about intermolecular forces.
Finally, it is of interest to point out that for those cases where a classical explanation exists (notably for electrostatic and induction forces) the quantum and the classical descriptions agree. This will be shown below.
General theory
From here on this article presupposes some knowledge of quantum mechanics, in particular the concept of antisymmetric wave functions and elementary perturbation theory.
The quantum mechanical basis for the majority of intermolecular effects is contained in a nonrelativistic energy operator, the molecular Hamiltonian. This operator consists only of kinetic energies and Coulomb interactions. Invoking the Born-Oppenheimer (BO) approximation, one considers the electronic (clamped nuclei) Hamilton operator only. The BO approximation allows the definition of an intermolecular potential energy. It is only by virtue of the BO approximation that one can consider the potential and its gradients with respect to different intermolecular degrees of freedom—the intermolecular forces.
For very long intermolecular distances the retardation of the Coulomb force (first considered in 1948 for intermolecular forces by Hendrik Casimir and Dirk Polder) may have to be included. Sometimes, e.g., for interacting paramagnetic or electronically excited molecules, electronic spin and other magnetic effects may play a role. In this article, however, retardation and magnetic effects will not be considered. The books[2][3] may be consulted for modern and thorough expositions of the theory of intermolecular forces.
It is common to distinguish four fundamental interactions, see e.g., Refs. [2][3][4][5]
- exchange
- electrostatic
- induction
- dispersion.
Perturbation theory
The last three of the fundamental interactions are most naturally accounted for by Rayleigh-Schrödinger perturbation theory (RS-PT). In this theory—applied to two monomers A and B—one uses as unperturbed Hamiltonian the sum of two monomer Hamiltonians,
while the perturbation is
where q i indicates the charge (in units e of elementary charge) of a particle of monomer A; q j belongs to monomer B. In the case of electrons q = −1, while a nucleus has q equal to its atomic number Z. The quantity r ij is the distance between particle i and particle j. Perturbation theory is based on expansions of perturbed states in terms of unperturbed states (eigenstates of H(0) ). In the present case the unperturbed states are products
Note on units: As is very common in quantum mechanical expositions, Gaussian units are applied here, that is, there appears no factor 1/(4πε0) in the Coulomb interaction. In this article the equations are further simplified by application of atomic units. Atomic units are derived from Gaussian units by putting Planck's reduced constant and the charge and mass of the electron equal to unity.
Supermolecular approach
The early theoretical work on intermolecular forces was invariably based on RS-PT and its antisymmetrized variants. However, since the beginning of the 1990s it has become possible to apply standard quantum chemical methods to pairs of molecules. This approach is referred to as the supermolecule method. In order to obtain reliable results one must include electronic correlation in the supermolecule method (without it dispersion is not accounted for at all), and take care of the basis set superposition error. This is the effect that the atomic orbital basis of one molecule improves the basis of the other. Since this improvement is distance dependent, it gives easily rise to artifacts.
Supermolecule calculations must be performed with very high precision, because the problem, known as weighing the captain, arises here. First one weighs the ship with the captain aboard (total energy of molecules in interaction) and then the ship is weighed with the captain ashore (total energy of molecules at an infinite distance apart); the difference gives the captain's weight. This analogy is due to the late Charles Coulson. The analogy is based on the fact that the total energy of molecules is six to seven orders of magnitude larger than a typical intermolecular interaction. That is, the significant digits in the results of supermolecule calculations start to appear beyond the sixth or seventh decimal place.
A disadvantage of the supermolecule method is that it yields the interaction as a lump sum. It does not give an interaction energy separated in the four fundamental contributions mentioned above. Therefore, the supermolecule will not be discussed method any further in this article.
Electron exchange
The monomer functions ΦnA and ΦmB are antisymmetric under permutation of electron coordinates (i.e., they satisfy the Pauli principle), but the product states are not antisymmetric under intermolecular exchange of the electrons. An obvious way to proceed would be to introduce the intermolecular antisymmetrizer . But, as already noticed in 1930 by Eisenschitz and London,[6] this causes two problems. In the first place the antisymmetrized unperturbed states are no longer eigenfunctions of H(0), which follows from the non-commutation
This non-commutation is due to the fact that in H(0) the electrons are assigned to a respective molecule, that is, the unperturbed Hamiltonian H(0) is not symmetric in all electrons. The total Hamiltonian HAB does contain all electrons symmetrically, which means that HAB is invariant under all permutations and commutes with the intermolecular antisymmetrizer. One can express this by stating that in HAB all electrons are indistinguishable, as they must be, whereas in H(0) they are only partly indistinguishable. Studies have shown[7] that the spectra (sets of energy eigenvalues) of H(0) and HAB are dramatically different. This means that the effect of the perturbation VAB is strong, even at large R. Conventional Rayleigh-Schrödinger perturbation theory is not capable to cope with this situation.
A second problem is that the projected excited states
become linearly dependent and there is no manifest choice of a physically meaningful linearly independent subset.
In the late 1960s, the Eisenschitz-London approach was revived and different rigorous variants of symmetry adapted perturbation theory were developed. (The word symmetry refers here to the permutational symmetry of electrons, i.e., to the indistinguishability of electrons). The different approaches shared a major drawback: they were very difficult to apply in practice. Hence a somewhat less rigorous approach (weak symmetry forcing) was introduced: apply ordinary RS-PT (Rayleigh-Schrödinger perturbation theory) and introduce the intermolecular antisymmetrizer at appropriate places in the RS-PT equations. This approach leads to feasible equations, and, when electronically correlated monomer functions are used, weak symmetry forcing gives reliable results.[4][5] The first-order (most important) energy including exchange is in almost all symmetry-adapted perturbation theories given by the following expression
An early application of this equation was by Walter Heitler and Fritz London who gave the first quantum mechanical account of covalent bonding (in H2). They presented, and evaluated numerically, this formula; see chemical bond. In the case of chemical bonding (as in H2) first order interactions (with functions in bra and ket adapted to proper permutation symmetry) are attractive for certain electron-spin states, usually spin-singlet and responsible for large bonding energies—on the order of a hundred kcal/mol. In the case of intermolecular forces between closed shell systems, the same kind of interaction is strongly repulsive and responsible for the "volume" of the molecule (see Van der Waals radius). Roughly speaking, the exchange interaction is proportional to the differential overlap between Φ0A and Φ0B. Since the wave functions decay exponentially as a function of distance, the exchange interaction does too. Hence the range of action is relatively short, which is why exchange interactions are referred to as short range interactions.
Electrostatic interactions
By definition the electrostatic interaction is given by the first-order Rayleigh-Schrödinger perturbation (RS-PT) energy (without exchange):
Within the clamped nuclei approximation, the clamped nucleus α on A has position vector Rα and its charge times the Dirac delta function, Zα δ(r—Rα), is the charge density of nucleus α. The total charge density ρAtot of monomer A is the sum of a nuclear contribution (sum of delta functions) and an electronic contribution (integral over electronic wave function squared):
with the electronic charge density given by an integral over nA − 1 primed electron coordinates:
An analogous definition holds for the charge density of monomer B. It can be shown that the first-order quantum mechanical expression can be written as
which is nothing but the classical expression for the electrostatic interaction between two charge distributions. This shows that the first-order RS-PT energy is indeed equal to the electrostatic interaction between A and B.
Multipole expansion
With present day computer soft- and hard-ware it is feasible to compute the electrostatic energy without any further approximations other than those applied in the computation of the monomer wave functions. In the past this was different and a further approximation was commonly introduced: VAB was expanded in a (truncated) series in inverse powers of the intermolecular distance RAB. This yields the multipole expansion of the electrostatic energy. Since its concepts still pervade the theory of intermolecular forces, it is presented here.
We will use the following vector notation
where θ and φ are the spherical polar angles of r with respect to a coordinate system in the laboratory (a so-called space-fixed frame). The quantity is in fact a unit vector along r and is defined by two polar angles. Further r is the length of r.
In this article the following expansion is proved
with the Clebsch-Gordan series defined by
The spherical harmonic in Racah normalization is defined by
The function YL,M is a to unity normalized spherical harmonic, while and are spherical multipole moment operators
where the sum over i is over electrons and nuclei of monomer A; their charges are given by q i. An equivalent definition holds for the multipole operators of monomer B.
Insertion of this expansion into the first-order (without exchange) expression gives a very similar expansion for the electrostatic energy, because the matrix element factorizes, Thus, the multipole-expansion of the electrostatic interaction (an expansion in powers of 1/RAB) is,
with the permanent multipole moments defined by the expectation values
The series is of infinite length, and, indeed, most molecules have an infinite number of non-vanishing multipoles. In the past, when computer calculations for the permanent moments were not yet feasible, it was common to truncate this series after the first non-vanishing term.
It depends very much on the symmetry of the monomers which terms are non-vanishing,. For instance, molecules with an inversion center such as a homonuclear diatomic (e.g., molecular nitrogen N2), or an organic molecule like ethene (C2H4) do not possess a permanent dipole moment (l = 1), but do carry a quadrupole moment (l = 2). Molecules such a hydrogen chloride (HCl) and water (H2O) lack an inversion center and hence do have a permanent dipole. So, the first non-vanishing electrostatic term in, e.g., the N2—H2O dimer, is the lA = 2, lB = 1 term. From the formula above follows that this term is of order L = lA + lB = 3 and has an R–4 dependence. Note, parenthetically, that in this dimer the quadrupole-quadrupole interaction (R–5) is not unimportant either, because the water molecule carries a non-vanishing quadrupole as well.
When computer calculations of permanent multipole moments of any order became possible, the matter of the convergence of the multipole series became more urgent. There are indications that for overlapping charge distributions the multipole expansion is divergent and is an asymptotic series.
Ionic interactions
It is debatable whether ionic interactions are to be seen as intermolecular forces, as some workers consider them rather as special kind of chemical bonding. The forces occur between charged atoms or molecules (ions). Ionic bonds are formed when the difference between the electron affinity of one monomer and the ionization potential of the other is so small in absolute value that electron transfer from the one monomer to the other is energetically favorable. Since a transfer of an electron is never complete there is always a degree of covalent bonding.
Once the ions (of opposite sign) are formed, the interaction between them can be seen as a special case of multipolar attraction, with a 1/RAB distance dependence. Indeed, the ionic interaction is the electrostatic term with lA = 0 and lB = 0. Using that the L = 0 term is simply 1/RAB and that the monopole moments and their Clebsch-Gordan coupling are
(where qA and qB are the charges of the molecular ions) we recover—as to be expected—Coulomb's law
For shorter distances, where the charge distributions of the monomers overlap, the ions will repel each other because of intermonomer exchange of the electrons.
Ionic compounds have high melting and boiling points due to the large amount of energy required to break the forces between the charged ions. When molten they are also good conductors of heat and electricity, due to the free or delocalised ions.
Numerical example
An example of a compound that is ionically bound is KCN. Due to the low ionization potential of potassium (4.339 eV) and the large electron affinity of CN (3.82 eV), the Coulomb attraction favors electron transfer from K to CN already at the large distance of 53 bohr (= 2.8 nm). For shorter distances the system can be regarded as to consist of two closed-shell monomers K+ and CN− that show the kind of intermolecular interactions discussed in this article. It can be estimated from numbers given in Ref.[8] that the dissociation energy of KCN → K + CN is about 75 kcal/mol (314 kJ/mol), which is the order of magnitude of a chemical binding energy. However, the motion of K+ around CN− is without appreciable energy barrier, whereas a covalent bond is characterized by a strong directionality. In that sense the K+ — CN− molecule indeed could be regarded as bound by intermolecular forces.
Dipole-dipole interactions
Dipole-dipole interactions, also called Keesom forces after Willem Hendrik Keesom, who gave the first mathematical description in 1921[9], are the forces that occur between two molecules with permanent dipoles. An example of this can be seen in hydrochloric acid:
The molecules are depicted here as two point dipoles in the energetically most unfavorable position. A point dipole is an idealization similar to a point charge (a finite charge in an infinitesimally small volume). A point dipole consists of two equal charges of opposite sign δ+ and δ– that are a distance d apart. The distance d is so small that at any distance R away from the point dipole, it holds that d/R >> (d/R)2. In this limit the electrostatic field outside the charge distribution consists of one (R−3) term only, see electric dipole. The electrostatic interaction between two point dipoles is given by the single term lA = 1 and lB = 1 in the expansion above.
Obviously, no molecule is an ideal point dipole, and in the case of the HCl dimer, for instance, dipole-quadrupole, quadrupole-quadrupole, etc., interactions are by no means negligible (and neither induction nor dispersion interactions can be neglected).
Note that the dipole-dipole interaction involving a closed-shell atom is zero, because a closed-shell atom does not carry a permanent dipole.
In order to obtain the mathematical equation for the dipole-dipole interaction, we must consider in the expansion of the electrostatic energy the term with lA = 1 and lB = 1. Because this expansion is termwise rotationally invariant, we can choose a convenient system of axes to evaluate the term. We choose a coordinate system centered on A with its z-axis coinciding with the intermolecular vector RAB. Under this circumstance it holds for Racah spherical harmonic that
Hence, the dipole-dipole term becomes after substitution of two Clebsch-Gordan coefficients
where
Analogous relations hold for the permanent dipole moments on B. Then
Introducing the dot product, we may write
and similarly for B, we get finally the well-known expression
Numerical example
As a numerical example we consider the HCl dimer depicted above. The left molecule is called A and the right B, so that the z-axis is along the molecules and points to the right. The present convention of the dipole vector is such that it points from negative to positive charge. [10] Hence (for clarity reasons we change the monomer label from superscript to subscript),
and it follows simply that
The value of μHCl is 0.43 (atomic units), so that at a distance of 10 bohr the dipole-dipole repulsion is 3.698 10–4 hartree (0.97 kJ/mol). If we rotate molecule B over 1800 degree, the negative chlorine atom comes close to the positive hydrogen atom of the other monomer and there is an attraction of 0.97 kJ/mol.
Thermal rotation
If one of the molecules is neutral and freely rotating, the total electrostatic interaction energy becomes zero, since all terms in the multipole expansion vanish upon free rotation, except the monopole-monopole interaction. (For the dipole-dipole interaction this is most easily proved by integrating over the spherical polar angles of the dipole vector, while using the volume element sinθ dθdφ). In gases and liquids the molecules are not rotating freely: for dipolar molecules the rotation is weighted by the Boltzmann factor exp(−Edip-dip/kT), where k is the Boltzmann constant and T the absolute temperature. It was first shown by W.H. Keesom [9] that the temperature-averaged dipole-dipole interaction is
Since the thermally averaged energy has an R−6 dependence, it is evidently much weaker than the unaveraged energy that has an R−3 dependence, but it is not completely zero. It is attractive, because the Boltzmann weighting favors somewhat the attractive regions of space. In HCl–HCl we find for T = 300 K and RAB = 10 bohr (0.53 nm) the averaged attraction −62 J/mol, which shows a weakening, due to thermal rotational motion, of the interaction by a factor of about 16. In the literature there is much confusion about the size of the dipole-dipole interaction, because some workers quote unaveraged values, while others quote thermally averaged values—the two differ by more than an order of magnitude.
Hydrogen bonding
Hydrogen bonding is an intermolecular interaction with a hydrogen atom being present in the intermolecular bond. This hydrogen is covalently (chemically) bound in one molecule, which acts as the proton donor. The other molecule acts as the proton acceptor. In the following important example of the water dimer, the water molecule on the right is the proton donor, while the one on the left is the proton acceptor:
The donated proton in a hydrogen bond is usually bound to an electronegative atom of the donor molecule. Examples of electronegative atoms are nitrogen, oxygen, and fluorine. The electronegative atom is negatively charged (carries a charge δ-) and the hydrogen atom bound to it is positively charged. Consequently the proton donor is a polar molecule with a relatively large dipole moment. Often the positively charged hydrogen atom points towards an electron rich region in the acceptor molecule. The fact that an electron rich region exists in the acceptor molecule, implies already that the acceptor has a relatively large dipole moment as well. The result is a dimer that to a large extent is bound by the dipole-dipole force.
For quite some time it was believed that hydrogen bonding required an explanation that was different from the other intermolecular interactions. However, reliable computer calculations that became possible since the 1980s, have shown that only the four effects listed above play a role, with the dipole-dipole interaction being singularly important. Since the four effects account completely for the bonding in small dimers like the water dimer, for which highly accurate calculations are feasible, it is now generally believed that no other bonding effects are operative.
Hydrogen bonds are found throughout nature. They give water its unique properties that are so important to life on earth. Hydrogen bonds between hydrogen atoms and nitrogen atoms of adjacent base pairs provide the intermolecular force that bind together the two strands in a molecule of DNA.
To give an indication of the order of magnitude of a typical hydrogen-bonding energy, we quote the estimate De = −5.00 kcal/mol = −20.9 kJ/mol for the equilibrium energy of the water dimer.[11]
Induction (polarization) forces
This type of interaction has a clear classical interpretation. Every molecule (except neutral S-state atom, such as the noble gases) offers an electric field to its surroundings. Every atom (including noble gas atoms) and every molecule is polarizable, that is, under influence of an electric field the charge distribution of the atom or molecule polarizes (positive charge shifts one way, negative charge the other, while the total charge is conserved). If we bring two molecules together, one molecule polarizes the other. This lowers the energy of the system and causes bonding. Generally speaking, induction energy is one of the less important components of the intermolecular force. It becomes important if one monomer has a monopole (charge) or large dipole, while the other is easily polarizable.
Quantum mechanically the induction interaction is described by second-order RS-PT (Rayleigh-Schrödinger perturbation theory). The RS-PT second-order energy is,
Here we assumed that both monomers have a discrete set of eigenstates. If not, the sums must be extended by integrals over the continuum part of the spectrum. Further we consider only the perturbation of the ground state, which we assume to be non-degenerate:
from which follows that the second-order energy E(2) is always negative. Finally, we restrict the double sum such that no "dangerous denominators" (i.e., zero denominators) appear. The double sum can be separated into three terms
In the first term on the right hand side A is in its ground state and polarizes B, in the second term B is in its ground state and polarizes A. The third term gives dispersion interaction and will be discussed in a later section.
It is possible to give general, non-multipole-expanded, expressions for the induction (without exchange). To this end we need to introduce a polarization propagator and to give an expression for the electric field generated by a molecule. [See, for instance, Eq. (19) in Ref.[4]]. It is also possible to use the multipole expansion and by an angular momentum recoupling procedure[12] to express the polarization energy in terms of monomer properties. Both approaches are outside the scope of this article.
A simple formula is obtained if both monomers are assumed to rotate freely. In contrast to the electrostatic energy, the rotationally averaged induction interaction is not zero, due to the negative definiteness of the second order RS-PT energy. We define a rotationally invariant and positive property, which is proportional to the squared length of the multipole moment,
and the generalized isotropic polarizability
The rotationally averaged induction energy is[12]
The most important term for neutral subsystems is for lA = lB = 1. The usual dipole polarizability α0 is proportional to the generalized isotropic polarizability and |M1|2 is proportional to the square of the length of the dipole μ:
where the dot product of μ with itself is introduced. The isotropic polarizability α0 is defined by
Hence the first induction terms are
As a numeric example we consider HCl again. Its polarizability α0 is 17.5 au. Polarization of one monomer by the dipole of the other at R = 10 bohr gives an attraction of 3.2 10−6 hartree, which corresponds to −8.5 J/mol. Twice this number (−17 J/mol) may be compared with the thermally averaged electrostatic energy −62 J/mol mentioned above.
London (dispersion) forces
In 1908 Kamerling Onnes and his coworkers liquified helium, which experimentally proved that two helium atoms attract each other—so that a liquid can be formed—but also that they repel each other—so that the liquid does not implode. Earlier (1873) Van der Waals had already assumed on theoretical grounds that there must be attraction and repulsion between atoms. He made this assumption in the derivation of his equation of state.
Until the advent of quantum mechanics it was an enigma why two noble gas atoms would repel or attract each other. Shortly after the introduction of the Schrödinger equation, Wang (1927) solved this equation perturbatively for two ground-state hydrogen atoms at large interatomic distance R. Approximating the electronic interaction by a Taylor series in 1/R he found an attractive potential with as leading term −C6/R6. A few years later (1930) London[13] showed that so-called oscillator strengths appear in the equations for the intermolecular interactions. Oscillator strengths were known from the classical theory of the dispersion of light developed by Drude and Lorentz. Also later, in the quantum mechanical dispersion theory of Kramers and Heisenberg, these quantities played a central role. Because of the similarity of his perturbation theory with the older dispersion theory, London coined the name dispersion effect for the attraction between noble gas atoms. It is common today to refer to these attractive long-range forces as London or dispersion forces.
The dispersion force acts between any two molecules and is clearly the only long-range interaction between two noble gas atoms, as noble gases do not have non-vanishing multipoles. No long range electrostatic or induction interactions are operative between noble gas atoms. After London's quantum mechanical account of the dispersion attraction, many workers have tried to find a classical explanation. So far this has not led to any quantitative mathematical expressions, but only to handwaving arguments in which instantaneous dipoles (vectors of undetermined direction and magnitude) play an important role.
The dimers that interact mainly through dispersion forces are usually very weakly bound. In the CO-dimer the equilibrium energy De is about 1.5 kJ/mol, but this value is not due to dispersion alone. Recall that the carbon monoxide molecule has a small dipole and a reasonable quadrupole, which contribute to the interaction as well.
For noble gas pairs, dispersion is the only attracting force. For instance, He2 has the equilibrium energy De = 0.091 kJ/mol. The dimer Ar2 has an equilibrium energy of 1.1 kJ/mol, which is more than an order of magnitude larger than the value of the helium dimer. As we will see below, this is due to the fact that the polarizability of the argon atom (11.09 bohr3) is much larger than that of the helium atom (1.38 bohr3). A large factor (∼ 48.2) appears also in the ratio of the C6 values of Ar2 and He2, which are 70.8 and 1.47 au, respectively. The ratio between the equilibrium energy of Ar2 and He2 (∼ 12.1) is less than would be expected from the ratio between their C6 values. This is because the equilibrium distance in He2 (5.6 bohr) is less than that in Ar2 (7.5 bohr), so that the C6/R6 term in the case of the helium dimer has a factor (7.5/5.6)6 = 5.8 more weight than in Ar2.
Quantum mechanical theory of dispersion forces
Above the quantum mechanical second-order RS-PT expression is given:
London developed the perturbation VAB in a series in R−1, where R is the distance between the nuclear centers of mass of the monomers. This series is the multipole expansion given above. When substituted into the second-order expression, Edisp is also obtained as series in R−1, with the first term being −C6/R6.
Much later (in the early 1980s) it was shown by several workers independently that the multipole expansion is not needed. It is possible to reformulate Edisp in terms of frequency-dependent polarization propagators. This work was a generalization of earlier work by Casimir and Polder (1948), who—using the multipole expansion—showed that the first term in the 1/R expansion can be written as the following integral
Here αA(iω) and αB(iω) are the frequency-dependent dipole polarizabilities of the respective atoms. The frequency ω is multiplied by the imaginary unit i. It is not difficult to give Casimir-Polder type expressions for the other terms in the expansion. In these higher terms one meets quadrupole, octupole, ... and mixed-pole (for example dipole-quadrupole) polarizabilities.
London obviously did not know the Casimir-Polder integral in 1930 and he made an additional approximation, named after Albrecht Unsöld. In this approximation one assumes that the excitation energies in the denominator of the second-order energies can be moved in front of the sum. In the summand remains an expression which is (almost) the unit operator—in its form of a resolution in terms of basis elements of Hilbert space (see closure relation). In this manner London obtained dispersion in terms of static (ω = 0) dipole polarizabilities and ionization potentials. He found the following approximation for the dispersion interaction Edisp between two atoms A and B. The quantities IA and IB are the first ionization potentials of the atoms,
Application of this London formula to the dispersion interaction between two hydrogen atoms [α(0) = 4 au, I = 0.5 hartree] gives C6 = 6 au. The accurate value is 6.499 au.
It was pointed out by Hirschfelder et al. in their authoritative treatise on intermolecular forces,[14] that no instantaneous dipoles are apparent in the present quantum mechanical formulation of dispersion. In the article dispersion interaction it is shown that an equation for E, of a similar form as the one above, appears in a time-independent quantum mechanical treatment of two oscillating dipoles that are interacting electrostatically.
Qualitatively the magnitude of a polarizability is a measure for the size of an atom or molecule. This is clearly demonstrated by the following dipole polarizabilities of the noble gases (in bohr3) from[15]
- He 1.383
- Ne 2.668
- Ar 11.09
- Kr 16.74
- Xe 27.0
Since the dispersion C6 values are proportional to dipole polarizabilities, the London dispersion forces are proportional to the sizes of the monomers as is shown by the following reasonably accurate upper bounds from Ref.[15] (C6 values in atomic units):
- He-He 1.47 Ne-Xe 46.5
- He-Ne 3.20 Ar-Ar 70.8
- He-Ar 10.1 Ar-Kr 100.
- He-Kr 14.2 Ar-Xe 151
- He-Xe 21.4 Kr-Kr 142
- Ne-Ne 7.27 Kr-Xe 215
- Ne-Ar 22.0 Xe-Xe 325
- Ne-Kr 30.9
Anisotropy and non-additivity of intermolecular forces
Consider the interaction between two electric point charges at position and . By Coulomb's law the interaction potential depends only on the distance between the particles. For molecules this is different. If we see a molecule as a rigid 3-D body, it has 6 degrees of freedom (3 degrees for its orientation and 3 degrees for its position in R3). The interaction energy of two molecules (a dimer) in isotropic and homogeneous space is in general a function of 2×6-6=6 degrees of freedom (by the homogeneity of space the interaction does not depend on the position of the center of mass of the dimer, and by the isotropy of space the interaction does not depend on the orientation of the dimer). The analytic description of the interaction of two arbitrarily shaped rigid molecules requires therefore 6 parameters. (One often uses two Euler angles per molecule, plus a dihedral angle, plus the distance.) The fact that the intermolecular interaction depends on the orientation of the molecules is expressed by stating that the potential is anisotropic. Since point charges are by definition spherical symmetric, their interaction is isotropic. Especially in the older literature, intermolecular interactions are regularly assumed to be isotropic (e.g., the potential is described in Lennard-Jones form, which depends only on distance).
Consider three arbitrary point charges at distances r12, r13, and r23 apart. The total interaction U is additive; i.e., it is the sum
Again for molecules this can be different. Pretending that the interaction depends on distances only—but see above—the interaction of three molecules takes in general the form
where is a non-additive three-body interaction. Such an interaction can be caused by[16] exchange interactions, by induction, and by dispersion (the latter is known as the Axilrod-Teller triple dipole effect).
References
- ↑ See H. Margenau and N. R. Kestner, Theory of Intermolecular Forces, Pergamon Press, Oxford, (1960) for an overview of the failed pre-quantum mechanical efforts in finding an explanation.
- ↑ 2.0 2.1 A. J. Stone, The Theory of Intermolecular Forces, 1996, Clarendon Press, Oxford
- ↑ 3.0 3.1 I. G. Kaplan, Intermolecular Interactions, Wiley, Hoboken NJ (2006)
- ↑ 4.0 4.1 4.2 B. Jeziorski, R. Moszynski, and K. Szalewicz, Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals complexes, Chemical Reviews, vol. 94, pp. 1887-1930 (1994). doi.
- ↑ 5.0 5.1 K. Szalewicz, K. Patkowski, and B. Jeziorski, Intermolecular interactions via perturbation theory: from diatoms to biomolecules, in: Structure and Bonding, vol. 116, pp. 43-117 (2005).
- ↑ R. Eisenschitz and F. London, Über das Verhältnis der van der Waalsschen Kräfte zu den homöopolaren Bindungskräften, (On the relation of the Van der Waals forces to the homopolar forces of bonding), Zeitschrift für Physik, vol. 60, p. 491 (1930). doi English translations in H. Hettema, Quantum Chemistry, Classic Scientific Papers, World Scientific, Singapore (2000), p. 336.
- ↑ K. Patkowski, T. Korona, and B. Jeziorski, Convergence Behavior of the Symmetry-Adapted Perturbation Theory for States Submerged in Pauli Forbidden Continuum, J. Chem. Phys. vol. 115, pp. 1137-1152 (2001).
- ↑ P. E. S. Wormer and J. Tennyson, J. Chem. Phys. vol 75, pp. 1245-1253 (1981)
- ↑ 9.0 9.1 W. H. Keesom, Phys. Zeitschrift, vol. 22 p. 129 (1921)
- ↑ This convention of direction of dipole, while common in physics, is opposite to the one used in organic chemistry. Since organic chemists hardly ever perform vector computations with dipoles, confusion hardly ever arises. In organic chemistry dipoles are mainly used in semi-quantitative discussions of charge separation in a molecule.
- ↑ E. M. Mass, et al., J. Chem. Phys. vol 113, pp. 6687-6701 (2000).
- ↑ 12.0 12.1 P. E. S. Wormer, F. Mulder, and A. van der Avoird, Quantum Theoretical Calculations of Van der Waals Interaction between Molecules. Anisotropic Long Range Interactions, International Journal of Quantum Chemistry, vol. 11, pp. 959-970 (1977)
- ↑ F. London, Zeitschrift für Physik, vol. 63, pp. 245-279 (1930) doi and Z. Physik. Chemie, vol. B11, p. 222 (1930). English translations in H. Hettema, Quantum Chemistry, Classic Scientific Papers, World Scientific, Singapore (2000).
- ↑ J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, Wiley, New York, (1954); page 955.
- ↑ 15.0 15.1 J. M. Standard and P. R. Certain, J. Chemical Physics, vol. 83, pp. 3002 - 3008 (1985)
- ↑ R. Moszynski, P. E. S. Wormer, B. Jeziorski, and A. van der Avoird, Symmetry-Adapted Perturbation Theory of Nonadditive Three-Body Interactions in van der Waals Molecules. I. General Theory, J. Chem. Phys. vol. 103, pp. 8058-8074 (1995)





![{\displaystyle {\big [}{\tilde {\mathcal {A}}}^{AB},H^{(0)}{\big ]}\equiv {\tilde {\mathcal {A}}}^{AB}H^{(0)}-H^{(0)}{\tilde {\mathcal {A}}}^{AB}\neq 0.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/353c1c7aab046a841368b1ca309963f822466ac1)








![{\displaystyle {\begin{aligned}V^{AB}&=\sum _{\ell _{A}=0}^{\infty }\sum _{\ell _{B}=0}^{\infty }(-1)^{\ell _{B}}{\binom {2\ell _{A}+2\ell _{B}}{2\ell _{A}}}^{1/2}\\&\times \sum _{M=-\ell _{A}-\ell _{B}}^{\ell _{A}+\ell _{B}}(-1)^{M}R^{-(\ell _{A}+\ell _{B}+1)}C_{\ell _{A}+\ell _{B},-M}({\widehat {\mathbf {R} }}_{AB})\;\left[\mathbf {Q} ^{\ell _{A}}\otimes \mathbf {Q} ^{\ell _{B}}\right]_{M}^{\ell _{A}+\ell _{B}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9a937e648bbc2f64a094443d9abeecd6c8defa53)
![{\displaystyle \left[\mathbf {Q} ^{\ell _{A}}\otimes \mathbf {Q} ^{\ell _{B}}\right]_{M}^{\ell _{A}+\ell _{B}}\equiv \sum _{m_{A}=-\ell _{A}}^{\ell _{A}}\sum _{m_{B}=-\ell _{B}}^{\ell _{B}}\;Q_{m_{A}}^{\ell _{A}}Q_{m_{B}}^{\ell _{B}}\;\langle \ell _{A},m_{A};\ell _{B},m_{B}|\ell _{A}+\ell _{B},M\rangle .}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6a695f5563a62a3dd78ff609eaf6580af2c6b4c8)
![{\displaystyle C_{L,M}({\widehat {\mathbf {R} }}_{AB})\equiv \left[{\frac {4\pi }{2L+1}}\right]^{1/2}\;Y_{L,M}({\widehat {\mathbf {R} }}_{AB}).}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c5edbea656971bc2c313136127a3db93334bb6a8)



![{\displaystyle {\begin{aligned}E_{\mathrm {electrostatic} }^{(1)}=&\sum _{\ell _{A}=0}^{\infty }\sum _{\ell _{B}=0}^{\infty }(-1)^{\ell _{B}}{\binom {2\ell _{A}+2\ell _{B}}{2\ell _{A}}}^{1/2}\,R_{AB}^{-(\ell _{A}+\ell _{B}+1)}\\&\times \sum _{M=-\ell _{A}-\ell _{B}}^{\ell _{A}+\ell _{B}}(-1)^{M}C_{\ell _{A}+\ell _{B},-M}({\widehat {\mathbf {R} }}_{AB})\;\left[\mathbf {M} ^{\ell _{A}}\otimes \mathbf {M} ^{\ell _{B}}\right]_{M}^{\ell _{A}+\ell _{B}},\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/330fcf8b14f902abbd68b873c73e8c315ba3fca1)

![{\displaystyle M_{0}^{0_{A}}=q_{A},\quad M_{0}^{0_{B}}=q_{B}\quad {\hbox{and}}\quad [\mathbf {M} ^{0_{A}}\otimes \mathbf {M} ^{0_{A}}]_{0}^{0}=q_{A}q_{B},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c8578235620d0c878c62a4121f8250ff0b27251f)

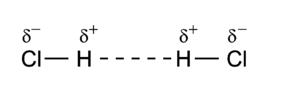

![{\displaystyle E_{\mathrm {dip-dip} }=-{\frac {1}{R_{AB}^{3}}}{\binom {4}{2}}^{1/2}\left[\mathbf {M} ^{1_{A}}\otimes \mathbf {M} ^{1_{B}}\right]_{0}^{2}=-{\frac {\sqrt {6}}{R_{AB}^{3}}}\left[{\frac {1}{\sqrt {6}}}(\mu _{1}^{A}\mu _{-1}^{B}+\mu _{-1}^{A}\mu _{1}^{B})+{\sqrt {\frac {2}{3}}}\mu _{0}^{A}\mu _{0}^{B}\right],}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a0e8f3abfeefd0a505b73af915e37cf78d2a8147)

![{\displaystyle E_{\mathrm {dip-dip} }={\frac {1}{R_{AB}^{3}}}\left[\mu _{x}^{A}\mu _{x}^{B}+\mu _{y}^{A}\mu _{y}^{B}-2\mu _{z}^{A}\mu _{z}^{B}\right].}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d45f09b37b3743bbcb4859d6b3b1cf6f158e4de0)

![{\displaystyle E_{\mathrm {dip-dip} }={\frac {1}{R_{AB}^{3}}}\left[{\boldsymbol {\mu }}^{A}\cdot {\boldsymbol {\mu }}^{B}-3({\boldsymbol {\mu }}^{A}\cdot {\hat {\mathbf {R} }}_{AB})({\hat {\mathbf {R} }}_{AB}\cdot {\boldsymbol {\mu }}^{B})\right].}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1e3aee8bd3cff1ff3aed93416e7a8dcff52accdd)