Streptococcus mutans: Difference between revisions
imported>Young Okeke |
mNo edit summary |
||
| (57 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{ | {{TOC|left}} | ||
{{Taxobox | |||
| color = pink | |||
| name = ''Streptococcus mutans'' | |||
| image = Streptococcus_mutans_01.jpg | |||
| image_width = 240px | |||
| regnum = Bacteria | |||
| phylum = Firmicutes | |||
| classis = Cocci | |||
| ordo = Lactobacillales | |||
| familia = Streptococcaceae | |||
| genus = Streptococcus | |||
| species = Mutans | |||
| binomial = ''Streptococcus mutans'' | |||
| binomial_authority = | |||
}} | |||
'''''Streptococcus mutans''''' was first described by JK Clark in 1924 after he isolated it from a [[dental caries|carious legion]]. <ref>{{citation | |||
| journal = Brit J Exp Pathol | |||
| author = J.K. Clark | |||
| title = On the bacterial factor in the etiology of dental caries | |||
| year = 1924}}</ref> Clarke also succeeded in producing caries in teeth in vitro with S. mutans, providing early evidence that S. mutans was a major cause of dental caries. Though discovered in the 1920's, it wasn’t until the 1960’s that a great deal of interest was generated when researchers began studying [[dental caries]] in detail. | |||
The group of oral streptococci closely related to S. mutans is referred to as the “mutans group” or mutans streptococci. The actual taxonomic bacteria Streptococcus mutans is always written in italics while the group name mutans streptococci is not. The mutans group consists of S. rattus, S. mutans, S. cricetus, S. maccacae, S. sobrinus, and S. downeii. S. mutans and S. sobrinus comprise the majority of the group and are only found in humans. They can be distinguished by laboratory tests, but it is not always practical due to costs and time. There is no selective media that would allow the detection and separation of the species and laboratory work is therefore done on the entire mutans streptococci group. Because of its greater prevalence, most of the isolates will in fact be S. mutans. [2][3] | |||
The | The two selective media that are widely used for isolating caries-related streptococci are based on Mitis-Salivarius agar and TYC agar to which the antibiotic Bacitracin is added (TYSCB). This suppresses the growth of most species but allows S. mutans and S. sobrinus to grow. The inclusion of sucrose leads to the formation of glucans and a distinctive colony appearance that aids in identification. [3] | ||
[ | One of the harmful effects of the normal flora, especially S. mutans, is bacterial synergism between a member of the normal flora and a potential pathogen. During the bacterial synergism, a member of the normal flora facilitates the growth of other potential pathogens. S. mutans facilitates such a condition through the initiation of the biofilm to which several oral bacteria adhere to and rely upon. S. mutans readily colonizes tooth surfaces and forms a thin film on the tooth called the enamel pellicle. It contains a cell-bound protein, glycosyl transferase, that serves as an adhesion for attachment to the tooth and produces lactic acid which demineralizes tooth enamel. Furthermore, if oral streptococci such as S. mutans are introduced into wounds created by dental manipulation or treatment, they may adhere to heart valves and initiate subacute bacterial endocarditis. [1] | ||
== | ==Genome structure== | ||
S. mutans has a single double stranded circular genome. The sequence of the 2,030,936 bp Streptococcus mutans strain UA159 Genome was completed and the results published in the October 29, 2002 issue of Proceedings National Academy of Sciences USA. This feat was accomplished first sequencing 12,000 individual, shotgun-based, double stranded templates. Next, a directed custom synthetic primer-based approach was used for closure and quality improvement to complete the sequence of the genome to a high level of accuracy. [5] | |||
==Cell structure and metabolism== | |||
S. mutans is an obligate anaerobe that receives energy through lactic acid fermentation. It is an alpha hemolytic Streptococci class bacteria that appears greenish on a blood agar plate. It possesses spherical cells that appear in chains due to cellular division in one plane and incomplete cytokinesis following mitosis. S. mutans is Gram-positive and catalase negative, preventing it from catalyzing the breakdown of hydrogen peroxide to oxygen and water. | |||
==Ecology== | |||
In utero the human fetal oral cavity is sterile, but the colonization of bacteria begins at birth. Handling and feeding of the infant after birth leads to the establishment of a stable normal flora in the oral cavity in about 48 hours. Streptococcus salivarius is the first dominant colonizer and may make up 98% of the total oral flora until the appearance of teeth occurs around 6 to 9 months of age. After the eruption of teeth, S. mutans and S. sanguis colonize the oral cavity and persist as long as teeth remain. | |||
S. mutans as well as other bacteria that compose the normal oral flora provide valuable services to the human host. They occupy available colonization sites which makes it more difficult for other microorganisms to establish themselves. The oral flora also contributes to host nutrition through the synthesis of vitamins, and they contribute to immunity by inducing low levels of circulating and secretory antibodies that have the potential to react with other pathogens. In addition, the oral flora exerts microbial antagonism against foreign species through the production of inhibitory substances. [1] | |||
==Pathology== | |||
S. mutans is normally present in the oral flora, but only becomes pathogenic under conditions that lead to frequent and prolonged acidic conditions. The cariogenic potential of S. mutans is manifested by the organism's ability to ferment various carbohydrates, producing large amounts of acid, and by its ability to participate in the formation of dental plaques. S. mutans is involved in the initial formation of the plaque biofilm through glucan formation. The dextran-like glucose polymers allows bacteria to stick to enamel of teeth to form a biofilm. This flora is extensive and may reach a thickness of 300-500 cells on the surface of the teeth. Acid production through lactic acid fermentation drives the dissolution of hydroxyapatite crystals and promotes the growth and development of acidic bacteria. Acid tolerance allows cariogenic bacteria to thrive and exclude bacteria that cannot survive in a pH below 5.5. [1][8][9] | |||
S. mutans is able to colonizes in high proportions through sucrose-dependent as well as sucrose-independent methods. Sucrose-independent adhesion involves both specific and nonspecific interactions with salivary glycoproteins and enamel pellicle. Sucrose-dependent adhesion relies on the synthesis of extracellular glucan polymers from sucrose by the action of glucosyltransferase enzymes. Both GbpB and GbpC are part of S. mutans cell wall and GbpB functions as a peptidoglycan hydrolase and may be necessary for cell wall cycling and synthesis. GbpC acts as a surface receptor for glucan and is responsible for dextran-dependent aggregation. GbpA and GbpD are secreted Gbps that contribute to sucrose-dependent biofilm production.[6] | |||
The effects of S. mutans can be counteracted through proper oral hygiene and the use of fluoride. Fluoride has been found to be the most effective agent against caries because it acts through topical mechanisms inhibiting the demineralization enamel and tooth structure, enhancement of remineralization at the crystal surfaces, and the inhibition of bacterial enzymes such as enolases, phosphatases, ATPases, and pyrophosphatases. Fluoride alters the physiochemical properteis of teeth by makging them more resistant to acid dissolution due to the formation of fluorapatite and fluorhydroxyapatite. [9] | |||
== | ==Application to Biotechnology== | ||
One application of S. mutans in biotechnology is the making paper utilizing glucans, produced by the glucosyltransferase C enzyme, instead of modified starches. Glucans are functionally similar to the hydroxethyl modified starch and are particularly useful in the coating step of paper manufacture. S. mutans or plants transformed with the gene encoding the glucosyltransferase C enzyme can be used to create a paper substitute and reduce the effects of deforestation and the clearing of trees for paper mining. [8] | |||
==Current Research== | |||
Manganese has been shown to be essential for the expression of S. mutans virulence factors such as the glucan-binding proteins. As such, current research is underway to examine the effects of Mn on the transcription of genes encoding Gbps. The glucan binding proteins (GbpA, GbpB, GbpC, and GbpD) promote adhesion and accumulation of S. mutans on tooth structures. [6] | |||
S. mutans | Researchers are working to interfere with key genes and proteins necessary for the survival of S. mutans to remove the ability of the bacteria to thrive in acidic conditions. Past research has shown that this ability has several components including the bacterial membrane bound enzyme fatty acid byosynthase M (FabM), which when shut down makes S. mutans 10,000 times more vulnerable to acid damage. In addition, early work suggests that FabM helps to resist the human body’s defenses. As a result, FabM is a major target for the design of new drugs. <ref>{{citation | ||
| url = http://www.sciencedaily.com/releases/2008/01/080102122300.htm | |||
| title = No Tooth Brush, No Cavities? Cavity-causing Bacteria May Be Made To Self-destruct | journal = ScienceDaily | date = 7 January 2008}}</ref> | |||
The effect of local immunization with Streptococcus mutans on dental caries is being studied with the hopes of creating a carries vaccine. It is proposed that salivary immunoglobulin A antibody may be viewed as an ecological determinant in the oral cavity by affecting oral microorganisms and possibly their by-products. [7] | |||
== | ==References== | ||
{{reflist}} | |||
[2] http://www.ncl.ac.uk/dental/oralbiol/oralenv/tutorials/streps.htm | |||
[3] http://www.ebi.ac.uk/2can/genomes/bacteria/Streptococcus_mutans.html | |||
[5] [http://www.genome.ou.edu/smutans.html Streptococcus mutans strain UA159 Genome Sequencing] | |||
== | [6] [http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowPDF&ArtikelNr=110883&Ausgabe=233772&ProduktNr=224219&filename=110883.pdfArirachakaran P, Benjavongkulchai E, Luengpailin S, Ajdicacute D, Banas JA: Manganese Affects Streptococcus mutans Virulence Gene Expression. Caries Res 2007;41:503-511 (DOI: 10.1159/000110883)] | ||
[7] [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=414936&blobtype=pdf Martin A. Taubman and Daniel J. Smith "Effects of Local Immunization with Streptococcus mutans on Induction of Salivary Immunoglobulin A Antibody and Experimental Dental Caries in Rats" Infect Immun. 1974 June; 9(6): 1079–1091.] | |||
[8] [http://www.patentstorm.us/patents/6127603.html Plant cells and plants transformed with streptococcus mutans gene encoding glucosyltransferase C enzyme] | |||
[9] [http://www.jisppd.com/temp/JIndianSocPedodPrevDent254157_235245.pdf Jeevarathan, J., Deepti, A., Muthu, M., Rathna Prabhu, V., Chamundeeswari, G. "Effect of fluoride varnish on Streptococcus mutans counts in plaque of caries-free children using dentocult SM strip mutans test: A randomized controlled triple blind study" Journal of Indian Society of Pedodontics and Preventive Dentistry. 2007. Volume 25 Issue 4 p. 157-163.][[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:01, 22 October 2024
| Streptococcus mutans | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
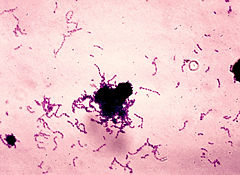 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Streptococcus mutans |
Streptococcus mutans was first described by JK Clark in 1924 after he isolated it from a carious legion. [1] Clarke also succeeded in producing caries in teeth in vitro with S. mutans, providing early evidence that S. mutans was a major cause of dental caries. Though discovered in the 1920's, it wasn’t until the 1960’s that a great deal of interest was generated when researchers began studying dental caries in detail.
The group of oral streptococci closely related to S. mutans is referred to as the “mutans group” or mutans streptococci. The actual taxonomic bacteria Streptococcus mutans is always written in italics while the group name mutans streptococci is not. The mutans group consists of S. rattus, S. mutans, S. cricetus, S. maccacae, S. sobrinus, and S. downeii. S. mutans and S. sobrinus comprise the majority of the group and are only found in humans. They can be distinguished by laboratory tests, but it is not always practical due to costs and time. There is no selective media that would allow the detection and separation of the species and laboratory work is therefore done on the entire mutans streptococci group. Because of its greater prevalence, most of the isolates will in fact be S. mutans. [2][3]
The two selective media that are widely used for isolating caries-related streptococci are based on Mitis-Salivarius agar and TYC agar to which the antibiotic Bacitracin is added (TYSCB). This suppresses the growth of most species but allows S. mutans and S. sobrinus to grow. The inclusion of sucrose leads to the formation of glucans and a distinctive colony appearance that aids in identification. [3]
One of the harmful effects of the normal flora, especially S. mutans, is bacterial synergism between a member of the normal flora and a potential pathogen. During the bacterial synergism, a member of the normal flora facilitates the growth of other potential pathogens. S. mutans facilitates such a condition through the initiation of the biofilm to which several oral bacteria adhere to and rely upon. S. mutans readily colonizes tooth surfaces and forms a thin film on the tooth called the enamel pellicle. It contains a cell-bound protein, glycosyl transferase, that serves as an adhesion for attachment to the tooth and produces lactic acid which demineralizes tooth enamel. Furthermore, if oral streptococci such as S. mutans are introduced into wounds created by dental manipulation or treatment, they may adhere to heart valves and initiate subacute bacterial endocarditis. [1]
Genome structure
S. mutans has a single double stranded circular genome. The sequence of the 2,030,936 bp Streptococcus mutans strain UA159 Genome was completed and the results published in the October 29, 2002 issue of Proceedings National Academy of Sciences USA. This feat was accomplished first sequencing 12,000 individual, shotgun-based, double stranded templates. Next, a directed custom synthetic primer-based approach was used for closure and quality improvement to complete the sequence of the genome to a high level of accuracy. [5]
Cell structure and metabolism
S. mutans is an obligate anaerobe that receives energy through lactic acid fermentation. It is an alpha hemolytic Streptococci class bacteria that appears greenish on a blood agar plate. It possesses spherical cells that appear in chains due to cellular division in one plane and incomplete cytokinesis following mitosis. S. mutans is Gram-positive and catalase negative, preventing it from catalyzing the breakdown of hydrogen peroxide to oxygen and water.
Ecology
In utero the human fetal oral cavity is sterile, but the colonization of bacteria begins at birth. Handling and feeding of the infant after birth leads to the establishment of a stable normal flora in the oral cavity in about 48 hours. Streptococcus salivarius is the first dominant colonizer and may make up 98% of the total oral flora until the appearance of teeth occurs around 6 to 9 months of age. After the eruption of teeth, S. mutans and S. sanguis colonize the oral cavity and persist as long as teeth remain.
S. mutans as well as other bacteria that compose the normal oral flora provide valuable services to the human host. They occupy available colonization sites which makes it more difficult for other microorganisms to establish themselves. The oral flora also contributes to host nutrition through the synthesis of vitamins, and they contribute to immunity by inducing low levels of circulating and secretory antibodies that have the potential to react with other pathogens. In addition, the oral flora exerts microbial antagonism against foreign species through the production of inhibitory substances. [1]
Pathology
S. mutans is normally present in the oral flora, but only becomes pathogenic under conditions that lead to frequent and prolonged acidic conditions. The cariogenic potential of S. mutans is manifested by the organism's ability to ferment various carbohydrates, producing large amounts of acid, and by its ability to participate in the formation of dental plaques. S. mutans is involved in the initial formation of the plaque biofilm through glucan formation. The dextran-like glucose polymers allows bacteria to stick to enamel of teeth to form a biofilm. This flora is extensive and may reach a thickness of 300-500 cells on the surface of the teeth. Acid production through lactic acid fermentation drives the dissolution of hydroxyapatite crystals and promotes the growth and development of acidic bacteria. Acid tolerance allows cariogenic bacteria to thrive and exclude bacteria that cannot survive in a pH below 5.5. [1][8][9]
S. mutans is able to colonizes in high proportions through sucrose-dependent as well as sucrose-independent methods. Sucrose-independent adhesion involves both specific and nonspecific interactions with salivary glycoproteins and enamel pellicle. Sucrose-dependent adhesion relies on the synthesis of extracellular glucan polymers from sucrose by the action of glucosyltransferase enzymes. Both GbpB and GbpC are part of S. mutans cell wall and GbpB functions as a peptidoglycan hydrolase and may be necessary for cell wall cycling and synthesis. GbpC acts as a surface receptor for glucan and is responsible for dextran-dependent aggregation. GbpA and GbpD are secreted Gbps that contribute to sucrose-dependent biofilm production.[6]
The effects of S. mutans can be counteracted through proper oral hygiene and the use of fluoride. Fluoride has been found to be the most effective agent against caries because it acts through topical mechanisms inhibiting the demineralization enamel and tooth structure, enhancement of remineralization at the crystal surfaces, and the inhibition of bacterial enzymes such as enolases, phosphatases, ATPases, and pyrophosphatases. Fluoride alters the physiochemical properteis of teeth by makging them more resistant to acid dissolution due to the formation of fluorapatite and fluorhydroxyapatite. [9]
Application to Biotechnology
One application of S. mutans in biotechnology is the making paper utilizing glucans, produced by the glucosyltransferase C enzyme, instead of modified starches. Glucans are functionally similar to the hydroxethyl modified starch and are particularly useful in the coating step of paper manufacture. S. mutans or plants transformed with the gene encoding the glucosyltransferase C enzyme can be used to create a paper substitute and reduce the effects of deforestation and the clearing of trees for paper mining. [8]
Current Research
Manganese has been shown to be essential for the expression of S. mutans virulence factors such as the glucan-binding proteins. As such, current research is underway to examine the effects of Mn on the transcription of genes encoding Gbps. The glucan binding proteins (GbpA, GbpB, GbpC, and GbpD) promote adhesion and accumulation of S. mutans on tooth structures. [6]
Researchers are working to interfere with key genes and proteins necessary for the survival of S. mutans to remove the ability of the bacteria to thrive in acidic conditions. Past research has shown that this ability has several components including the bacterial membrane bound enzyme fatty acid byosynthase M (FabM), which when shut down makes S. mutans 10,000 times more vulnerable to acid damage. In addition, early work suggests that FabM helps to resist the human body’s defenses. As a result, FabM is a major target for the design of new drugs. [2]
The effect of local immunization with Streptococcus mutans on dental caries is being studied with the hopes of creating a carries vaccine. It is proposed that salivary immunoglobulin A antibody may be viewed as an ecological determinant in the oral cavity by affecting oral microorganisms and possibly their by-products. [7]
References
- ↑ J.K. Clark (1924), "On the bacterial factor in the etiology of dental caries", Brit J Exp Pathol
- ↑ "No Tooth Brush, No Cavities? Cavity-causing Bacteria May Be Made To Self-destruct", ScienceDaily, 7 January 2008
[2] http://www.ncl.ac.uk/dental/oralbiol/oralenv/tutorials/streps.htm
[3] http://www.ebi.ac.uk/2can/genomes/bacteria/Streptococcus_mutans.html