Warfarin: Difference between revisions
imported>Robert Badgett |
mNo edit summary |
||
| (57 intermediate revisions by 4 users not shown) | |||
| Line 4: | Line 4: | ||
'''Warfarin''' (IUPAC name | '''Warfarin''' (IUPAC name | ||
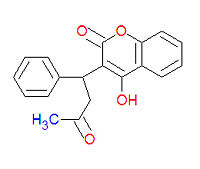
4-hydroxy-3-(3-oxo-1-phenylbutyl)-2H-chromen-2-one), also widely called coumadin, is | 4-hydroxy-3-(3-oxo-1-phenylbutyl)-2H-chromen-2-one), also widely called coumadin, is an [[anticoagulant]] medication used prophylactically to suppress the formation of [[embolism and thrombosis]] from conditions such as [[atrial fibrillation]], [[deep venous thrombosis]], and [[pulmonary embolism]]. | ||
Originally designed to be a rat poison, warfarin works as an anticoagulant by suppressing the enzyme [[epoxide reductase]] in the liver, thereby suppressing the formation of the reduced form of [[vitamin K]] epoxide, which is needed for the synthesis of many coagulation factors. As a drug, it is often sold as the sodium salt of warfarin. | |||
==Discovery== | ==Discovery== | ||
The dangers of feeding livestock spoiled sweet clover hay were known in the 1920’s<ref> | The dangers of feeding livestock spoiled sweet clover hay were known in the 1920’s<ref>Wardrop D, Keeling D (2008) The story of the discovery of heparin and warfarin ''Brit J Haematol'' 141:757-63</ref> and scientists at the [[University of Wisconsin-Madison]] were spearheading the problem on multiple fronts. R.A. Brink and W.K. Smith were attempting to breed a new variety of clover that was free of the toxic effect and [[Karl Paul Link]]'s laboratory was attempting to isolate the killer compound. Farmers were already hurting due to the [[Great Depression]] and despite knowing they should not feed their livestock such hay, they could not afford to buy uncontaminated supplies. This disease that was affecting many livestock throughout the US became known as "sweet clover disease".<ref>Duxbury BM, Poller L (2001) The oral anticoagulant saga: past, present, and future ''Clin Appl Thrombosis/Hemostasis'' 7:269–75 PMID 11697707</ref> | ||
The urgency of this work was punctuated in the winter of 1933 when Ed Carlson, a farmer from Deer Park, Wisconsin, came to Madison for help carrying a milk can of uncoagulated blood, a dead cow in his truck and a sample of the hay contaminated with the spoiled sweet clover. This spurred Link on and in 1940 he finally published the source of the hemorrhagic factor in sweet clover, 3,3'-methylene-bis[4-hyfroxycoumarin], known as coumarin.<ref>Campbell | The urgency of this work was punctuated in the winter of 1933 when Ed Carlson, a farmer from Deer Park, Wisconsin, came to Madison for help carrying a milk can of uncoagulated blood, a dead cow in his truck and a sample of the hay contaminated with the spoiled sweet clover. This spurred Link on and in 1940 he finally published the source of the hemorrhagic factor in sweet clover, 3,3'-methylene-bis[4-hyfroxycoumarin], known as coumarin.<ref>Campbell HA ''et al.'' (1940) Studies on the hemorrhagic sweet clover disease. I. The preparation of hemorrhagic concentrates. ''J Biol Chem'', '''136''', 47–55.</ref> The following year Link published the discovery of dicumarol, the oxidised form of coumarin that was present in the spoiled sweet clover; this became widely used as an oral anticoagulants for medical treatment.<ref>Campbell HA ''et al.'' (1941) Studies on the hemorrhagic sweet clover disease. II. The bioassay of hemorrhagic concentrates by following the prothrombin level in the plasma of rabbit blood. ''J Biol Chem'' 138:1–20</ref><ref>Stahmann MA ''et al.'' (1941) Studies on the hemorrhagic sweet clover disease. V. Identification and synthesis of the hemorrhagic agent ''J Biol Chem'' 138:513–27</ref> | ||
Given the death of cattle from hemorrhaging, Link began to realise that anticoagulants had potential to be rodenticides. | Given the death of cattle from hemorrhaging, Link began to realise that anticoagulants had potential to be rodenticides. | ||
| Line 15: | Line 17: | ||
I had an intuitive feeling that this might be a good thing. A pretty bad thing for rats, but a good thing for humans. But the idea didn't come overnight. It came into my head and the heads of everyone in the lab over a period of years. <ref>Karl Paul Link, [http://www.warf.org/about/index.jsp?cid=26&scid=34 Societal Contributions] hosted by Wisconsin Alumni Research Foundation</ref> | I had an intuitive feeling that this might be a good thing. A pretty bad thing for rats, but a good thing for humans. But the idea didn't come overnight. It came into my head and the heads of everyone in the lab over a period of years. <ref>Karl Paul Link, [http://www.warf.org/about/index.jsp?cid=26&scid=34 Societal Contributions] hosted by Wisconsin Alumni Research Foundation</ref> | ||
</blockquote> | </blockquote> | ||
Dicumarol turned out to be a poor rodenticide as it acted too slowly<ref>Last | Dicumarol turned out to be a poor rodenticide as it acted too slowly<ref>Last JA (2002) The missing link: the story of Karl Paul Link. ''Toxicol Sci'' 66:4–6.</ref> but by 1948 Link had patented another coumarin derivative as a rodenticide. Research for both dicumarol and the new rodenticide was funded by the [[Wisconsin Alumni Research Foundation]] (WARF) and the [[Warfarin/Catalogs|brand name]] for these anticoagulating rat posions was coined 'Warfarin' after WARF. Later, the rodenticide was promoted for clinical applications under the brand name 'Coumadin'. | ||
==Mechanism of action== | ==Mechanism of action== | ||
Warfarin therapy reduces the [[vitamin K]] dependent cofactors II, VII, IX, and X and the [[vitamin K]] dependent [[Protein C]]. The level of factor II is thought to most influence coagulation.<ref name="pmid15522981">{{cite journal |author=Eckhoff CD, Didomenico RJ, Shapiro NL |title=Initiating warfarin therapy: 5 mg versus 10 mg |journal=Ann Pharmacother |volume=38 | Warfarin therapy reduces the [[vitamin K]] dependent cofactors II, VII, IX, and X and the [[vitamin K]] dependent [[Protein C]]. The level of factor II is thought to most influence coagulation.<ref name="pmid15522981">{{cite journal |author=Eckhoff CD, Didomenico RJ, Shapiro NL |title=Initiating warfarin therapy: 5 mg versus 10 mg |journal=Ann Pharmacother |volume=38 |pages=2115–21 |year=2004 |pmid=15522981 |doi=10.1345/aph.1E083}}</ref><ref name="pmid9005747">{{cite journal |author=Harrison L ''et al.'' |title=Comparison of 5-mg and 10-mg loading doses in initiation of warfarin therapy |journal=Ann Intern Med |volume=126 |pages=133–6 |year=1997 |pmid=9005747 |doi= |issn=|url=http://www.annals.org/cgi/content/full/126/2/133}}</ref> The levels of factor VII and [[Protein C]] fall the fastest after warfarin is started.<ref name="pmid9005747"/> With the exception of Factor IX, these factors are from either the extrinsic pathway or the final common pathway. | ||
==Pharmacokinetics== | ==Pharmacokinetics== | ||
| Line 27: | Line 27: | ||
===Metabolism=== | ===Metabolism=== | ||
====Pharmacogenomics==== | ====Pharmacogenomics==== | ||
About 15% of the effect of warfarin can be explained by the amount of warfarin in the blood.<ref name="pmid7586953">{{cite journal| author=White RH, Zhou H, Romano P, Mungall D| title=Changes in plasma warfarin levels and variations in steady-state prothrombin times. | journal=Clin Pharmacol Ther | year= 1995 | volume= 58 | issue= 5 | pages= 588-93 | pmid=7586953 | doi=10.1016/0009-9236(95)90179-5 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7586953 }} </ref> About 50% of the effect of warfarin can be explained by genetic factors.<ref name="pmid18305455">{{cite journal| author=Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM et al.| title=Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. | journal=Clin Pharmacol Ther | year= 2008 | volume= 84 | issue= 3 | pages= 326-31 | pmid=18305455 | doi=10.1038/clpt.2008.10 | pmc=PMC2683977 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18305455 }} </ref><ref name="pmid15947090">{{cite journal| author=Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP et al.| title=The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. | journal=Blood | year= 2005 | volume= 106 | issue= 7 | pages= 2329-33 | pmid=15947090 | doi=10.1182/blood-2005-03-1108 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15947090 }} </ref><ref name="pmid19686083">{{cite journal| author=Kamali F, Wynne H| title=Pharmacogenetics of warfarin. | journal=Annu Rev Med | year= 2010 | volume= 61 | pages= 63-75 | pmid=19686083 | }} </ref> The American [[Food and Drug Administration]] "highlights the opportunity for healthcare providers to use genetic tests to improve their initial estimate of what is a reasonable warfarin dose for individual patients".<ref>{{cite web |url=http://www.fda.gov/bbs/topics/NEWS/2007/NEW01684.html |title=FDA Approves Updated Warfarin (Coumadin) Prescribing Information |accessdate=2007-08-20 |format= |work=}}</ref> A [[systematic review]] concluded that as of early 2009, there is insufficient benefit in genetic testing.<ref name="pmid19306050">{{cite journal |author=Kangelaris KN ''et al.'' |title=Genetic testing before anticoagulation? A systematic review of pharmacogenetic dosing of warfarin |journal=J Gen Intern Med |volume=24 |pages=656–64 |year=2009|pmid=19306050 }}</ref> | |||
=====VKORC1===== | =====VKORC1===== | ||
[[Genetic polymorphism]]s in the ''[[vitamin K epoxide reductase]] complex 1 (VKORC1)'' gene explain 30% of the dose variation between patients<ref name="pmid15883587">{{cite journal |author=Wadelius M | [[Genetic polymorphism]]s in the ''[[vitamin K epoxide reductase]] complex 1 (VKORC1)'' gene explain 30% of the dose variation between patients<ref name="pmid15883587">{{cite journal |author=Wadelius M ''et al'' |title=Common VKORC1 and GGCX polymorphisms associated with warfarin dose |journal=Pharmacogenomics J |volume=5 |pages=262-70 |year=2005 |pmid=15883587 }}</ref>: particular mutations make VKORC1 less susceptible to suppression by warfarin<ref name="pmid14765194">{{cite journal |author=Rost S ''et al'' |title=Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2 |journal=Nature |volume=427 |pages=537–41 |year=2004 |pmid=14765194 }}</ref> There are a main haplotypes that explain 25% of variation: low-dose haplotype group (A) and a high-dose haplotype group (B).<ref name="pmid15930419">{{cite journal |author=Rieder MJ ''et al'' |title=Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose |journal=N Engl J Med |volume=352 |pages=2285-93 |year=2005 |pmid=15930419 }}</ref> For the three combinations of the haplotypes, the mean daily maintenance dose of warfarin was: | ||
* A/A: 2.7+/-0.2 mg | * A/A: 2.7+/-0.2 mg | ||
* A/B: 4.9+/-0.2 mg | * A/B: 4.9+/-0.2 mg | ||
* B/B: 6.2+/-0.3 mg | * B/B: 6.2+/-0.3 mg | ||
''VKORC1'' polymorphisms also explain why African Americans are relatively resistant to warfarin (higher proportion of group B haplotypes), while Asian Americans are more sensitive (higher proportion of group A haplotypes).<ref name="pmid15930419" | ''VKORC1'' polymorphisms also explain why African Americans are relatively resistant to warfarin (higher proportion of group B haplotypes), while Asian Americans are more sensitive (higher proportion of group A haplotypes).<ref name="pmid15930419"/> | ||
=====CYP2C9===== | =====CYP2C9===== | ||
''CYP2C9'' is an [[isoenzyme]] of [[cytochrome P-450]]. [[Genetic polymorphism|Polymorphisms]] of CYP2C9 explain another 10% of variation in warfarin dosing<ref name="pmid15883587"/>, mainly among Caucasian patients as these variants are rare in African American and most Asian populations.<ref name="pmid15714076">{{cite journal |author=Sanderson S, Emery J, Higgins J |title=CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis |journal=Genet | ''CYP2C9'' is an [[isoenzyme]] of [[cytochrome P-450]]. [[Genetic polymorphism|Polymorphisms]] of CYP2C9 explain another 10% of variation in warfarin dosing<ref name="pmid15883587"/>, mainly among Caucasian patients as these variants are rare in African American and most Asian populations.<ref name="pmid15714076">{{cite journal |author=Sanderson S, Emery J, Higgins J |title=CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis |journal=Genet Med |volume=7 |pages=97-104 |year=2005 |pmid=15714076 }}</ref> A [[meta-analysis]] of mainly Caucasian patients found<ref name="pmid15714076"/>: | ||
* CYP2C9*2 allele: | * CYP2C9*2 allele: | ||
** present in 12.2% of patients | ** present in 12.2% of patients | ||
| Line 49: | Line 48: | ||
** relative bleeding risk was 1.77 | ** relative bleeding risk was 1.77 | ||
===Excretion=== | |||
=== | ==Availability== | ||
Generic preparations have similar pharmacology.<ref name="pmid21449627">{{cite journal| author=Dentali F ''et al.''| title=Brand name versus generic warfarin: a systematic review of the literature. | journal=Pharmacotherapy | year= 2011 | volume= 31 | issue= 4 | pages= 386-93 | pmid=21449627 }} </ref> | |||
==Dosage== | ==Dosage== | ||
[[Clinical practice guideline]]s are available for using warfarin.<ref name="pmid22315259">{{cite journal| author=Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ et al.| title=Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. | journal=Chest | year= 2012 | volume= 141 | issue= 2 Suppl | pages= e152S-84S | pmid=22315259 | doi=10.1378/chest.11-2295 | pmc=PMC3278055 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22315259 }} </ref> | |||
"After 4 to 5 days of concomitant warfarin and heparin therapy, heparin is discontinued when the INR has been in the therapeutic range on two measurements approximately 24 h apart."<ref name="pmid18574265">{{cite journal| author=Ansell J ''et al.''| title=Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) | journal=Chest | year= 2008 | volume= 133 Suppl | pages= 160S-98S | pmid=18574265 | |||
}}</ref> | |||
===Loading regimens=== | ===Loading regimens=== | ||
A [[decision analysis]] "suggests that patients with intermediate and high/likely probabilities of [[pulmonary embolism|PE]] benefit from preemptive [[anticoagulation]]". <ref name="pmid22383664">{{cite journal| author=Blondon M, Righini M, Aujesky D, Le Gal G, Perrier A| title=Utility of preemptive anticoagulation in patients with suspected pulmonary embolism: a decision analysis. | journal=Chest | year= 2012 | volume= | issue= | pages= | pmid=22383664 | doi=10.1378/chest.11-2694 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22383664 }} </ref> | |||
Because of warfarin's difficult [[pharmacokinetics]], researchers have proposed algorithms for warfarin loading. | Because of warfarin's difficult [[pharmacokinetics]], researchers have proposed algorithms for warfarin loading. | ||
{| class="wikitable" | {| class="wikitable" | ||
|+ [[Randomized controlled trial]]s of warfarin loading algorithms<ref name="pmid12729425">{{cite journal |author=Kovacs MJ, Rodger M, Anderson DR, ''et al'' |title=Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial |journal=Ann | |+ [[Randomized controlled trial]]s of warfarin loading algorithms<ref name="pmid12729425">{{cite journal |author=Kovacs MJ, Rodger M, Anderson DR, ''et al'' |title=Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial |journal=Ann Intern Med|volume=138|pages=714-9 |year=2003 |pmid=12729425 |doi=|url=http://annals.org/cgi/content/full/138/9/714}}</ref><ref name="pmid9005747"/><ref name="pmid9892329">{{cite journal |author=Crowther MA ''et al.'' |title=A randomized trial comparing 5-mg and 10-mg warfarin loading doses |journal=Arch Intern Med|volume=159 |pages=46–8 |year=1999 |pmid=9892329}}</ref><ref name="pmid16893712">{{cite journal |author=Quiroz R ''et al.'' |title=Comparison of a single end point to determine optimal initial warfarin dosing (5 mg versus 10 mg) for venous thromboembolism |journal=Am J Cardiol |volume=98|pages=535–7 |year=2006 |pmid=16893712 }}</ref><ref name="pmid17989110">{{cite journal |author=Anderson JL ''et al.'' |title=Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation |journal=Circulation |volume=116|pages=2563–70 |year=2007 |pmid=17989110 }}</ref><ref name="pmid21035980">{{cite journal| author=Farahmand S, Saeedi M, Seyed Javadi HH, Khashayar P| title=High doses of warfarin are more beneficial than its low doses in patients with deep vein thrombosis. | journal=Am J Emerg Med | year= 2011 | volume= 29 | issue= 9 | pages= 1222-6 | pmid=21035980 | doi=10.1016/j.ajem.2010.07.008 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21035980 }} </ref><ref name="pmid17851566">{{cite journal |author=Caraco Y ''et al.'' |title=CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study |journal=Clin Pharmacol Ther |volume=83 |pages=460–70 |year=2008 |pmid=17851566 }}</ref> | ||
! !! Dosing !!Study !! Time till a therapeutic INR<br/>(days)!! Rate of anticoagulation<br>(INR=2-3)!! Rate of over-coagulation | ! !! Dosing !!Study !! Time till a therapeutic INR<br/>(days)!! Rate of anticoagulation<br>(INR=2-3)!! Rate of over-coagulation | ||
|- | |- | ||
| rowspan=" | | rowspan="4" style="background:lightgreen"| Kovacs 10 mg || rowspan="4" style="background:lightgreen"| 10 mg/day for two days then<br>specified [http://www.aafp.org/afp/2005/0215/p763.html#afp20050215p763-f1 adjusted dosing]||Kovacs†<ref name="pmid12729425"/>||align="center"|4.2 || 83% by day 5 || 9% ''within 4 weeks''(INR>5)<br>(most occurred after day 10) | ||
|- | |- | ||
| Quiroz‡<ref name="pmid16893712"/>||align="center"|5<br/>(two consecutive INRs≥2)||56% at 5 days || 0% within 5 days (INR>5) | | Quiroz‡<ref name="pmid16893712"/><br/>Mean age: 51<br/>United States||align="center"|5<br/>(two consecutive INRs≥2)||56% at 5 days || 0% within 5 days (INR>5) | ||
|- | |- | ||
| Anderson‡<ref name="pmid17989110"/>||align="center"| ||69% at 5 days || | | Anderson‡<ref name="pmid17989110"/>||align="center"| ||69% at 5 days || | ||
|- | |- | ||
| rowspan="2" | Harrison 10 mg || rowspan="2" | 10 mg/day for one day then<br>flexible [http:// | | Farahmand‡<ref name="pmid21035980"/><br/>Mean age: 48<br/>Iran||align="center"| ||27% at 5 days || 2% at 5 days (INR>5) | ||
|- | |||
| rowspan="2" | Harrison 10 mg || rowspan="2" | 10 mg/day for one day then<br>flexible [http://annals.org/article.aspx?articleid=710776 adjusted dosing]|| Harrison¶<ref name="pmid9005747"/>|| ||63% at 3.5 days || 17% within 3.5 days (INR>4.8) | |||
|- | |- | ||
| Crowther¶<ref name="pmid9892329"/>||align="center"| ||69% at 5 days || 0% within 5 days (INR>5)<br>24% within 5 days (INR>3) | | Crowther¶<ref name="pmid9892329"/>||align="center"| ||69% at 5 days || 0% within 5 days (INR>5)<br>24% within 5 days (INR>3) | ||
|- | |- | ||
| Kovacs 5 mg || 5 mg/day for two days then<br>specified [http:// | | rowspan="2" |Kovacs 5 mg ||rowspan="2" | 5 mg/day for two days then<br>specified [http://www.aafp.org/afp/2005/0215/p763.html#afp20050215p763-t1 adjusted dosing]|| Kovacs†<ref name="pmid12729425"/>||align="center"|5.6|| 46% by day 5 || 11% ''within 4 weeks'' (INR>5)<br>(most occurred after day 10) | ||
|- | |- | ||
| style="background:lightgreen" rowspan="3"|Harrison 5 mg ||rowspan="3" style="background:lightgreen"| 5 mg/day for one day then<br>flexible [http:// | | Farahmand‡<ref name="pmid21035980"/><br/>Mean age: 48<br/>Iran||align="center"| ||47% at 5 days || 2% at 5 days (INR>5) | ||
|- | |||
| style="background:lightgreen" rowspan="3"|Harrison 5 mg ||rowspan="3" style="background:lightgreen"| 5 mg/day for one day then<br>flexible [http://annals.org/article.aspx?articleid=710776 adjusted dosing]||Harrison¶ <ref name="pmid9005747"/>||align="center"| || 80% at 3.5 days || 4% within 4.5 days (INR>4.8) | |||
|- | |- | ||
| Crowther¶<ref name="pmid9892329"/>||align="center"| ||88% at 5 days || 3% within 5 days (INR>5)<br>7% within 5 days (INR>3) | | Crowther¶<ref name="pmid9892329"/>||align="center"| ||88% at 5 days || 3% within 5 days (INR>5)<br>7% within 5 days (INR>3) | ||
|- | |- | ||
| Quiroz‡<ref name="pmid16893712"/>||align="center"|5<br/>(two consecutive INRs≥2)||52% at 5 days || 0% within 5 days (INR>5) | | Quiroz‡<ref name="pmid16893712"/><br/>Mean age: 51<br/>United States||align="center"|5<br/>(two consecutive INRs≥2)||52% at 5 days || 0% within 5 days (INR>5) | ||
|- | |- | ||
| Anderson [[Pharmacogenomics|pharmacogenetic]]-guided algorithm || Based on ''[[CYP2C9]]'' and ''[[VKORC1]]''|| Anderson†<ref name="pmid17989110"/>||align="center"| ||70% at 5 days|| | | Anderson [[Pharmacogenomics|pharmacogenetic]]-guided algorithm || Based on ''[[CYP2C9]]'' and ''[[VKORC1]]''|| Anderson†<ref name="pmid17989110"/>||align="center"| ||70% at 5 days|| | ||
| Line 84: | Line 95: | ||
|colspan="6"|† Blinded study.<br/>‡ Independent study not from original investigators.<br/>¶ Same research group (Hamilton, Ontario).<br/> | |colspan="6"|† Blinded study.<br/>‡ Independent study not from original investigators.<br/>¶ Same research group (Hamilton, Ontario).<br/> | ||
'''Notes''': | '''Notes''': | ||
<br/>1. INR. [[International Normalized Ratio]]<br>2. The Kovacs 5 mg algorithm is the same as the Harrison 5 mg algorithm except that where Harrison gave a range warfarin of dosages based on the INR, Kovacs specified a dose (usually at the high end of the range offered by Harrison).<ref name="pmid15522981" | <br/>1. INR. [[International Normalized Ratio]]<br>2. The Kovacs 5 mg algorithm is the same as the Harrison 5 mg algorithm except that where Harrison gave a range warfarin of dosages based on the INR, Kovacs specified a dose (usually at the high end of the range offered by Harrison).<ref name="pmid15522981"/> | ||
|} | |} | ||
| Line 90: | Line 101: | ||
On first look, the evidence table suggests that the Harrison 5 mg algorithm from Hamilton is the chest combination of efficacy and safety; however, two independent studies (Kovacs<ref name="pmid12729425"/> and Quiroz in 2006 from the Massachusetts General Hospital<ref name="pmid16893712"/>) have not been able to replicate the results of Hamilton group of Harrison and Crowther. One explanation may be that the personnel in the Harrison study were more expert and the flexibility in the algorithm allowed expression of their expertise. If so, then perhaps equally expert health care providers should use the Harrison 5 mg algorithm while other personnel should use the Kovacs 10 mg algorithm. Considering that the expertise of the Massachusetts General Hospital could not replicate the Hamilton results, perhaps most providers should use the Kovacs 10 mg algorithm, at least for inpatients. | On first look, the evidence table suggests that the Harrison 5 mg algorithm from Hamilton is the chest combination of efficacy and safety; however, two independent studies (Kovacs<ref name="pmid12729425"/> and Quiroz in 2006 from the Massachusetts General Hospital<ref name="pmid16893712"/>) have not been able to replicate the results of Hamilton group of Harrison and Crowther. One explanation may be that the personnel in the Harrison study were more expert and the flexibility in the algorithm allowed expression of their expertise. If so, then perhaps equally expert health care providers should use the Harrison 5 mg algorithm while other personnel should use the Kovacs 10 mg algorithm. Considering that the expertise of the Massachusetts General Hospital could not replicate the Hamilton results, perhaps most providers should use the Kovacs 10 mg algorithm, at least for inpatients. | ||
A [[systematic review]] of the [[randomized controlled trial]]s done through 2003 of 5 mg versus the 10 mg concluded that the Kovacs 10 mg regimen is best.<ref name="pmid15522981" | A [[systematic review]] of the [[randomized controlled trial]]s done through 2003 of 5 mg versus the 10 mg concluded that the Kovacs 10 mg regimen is best.<ref name="pmid15522981"/> This conclusion was largely based on the inability of the results of the Harrison 5 mg flexible algorithm to be replicated by Kovacs. | ||
[[Clinical practice guideline]]s in 2004 by the [[American College of Chest Physicians]] concluded that either 5 or 10 loads are acceptable.<ref name="pmid15383473">{{cite journal |author=Ansell J | [[Clinical practice guideline]]s in 2004 by the [[American College of Chest Physicians]] concluded that either 5 or 10 loads are acceptable.<ref name="pmid15383473">{{cite journal |author=Ansell J ''et al.'' |title=The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy |journal=Chest |volume=126 |issue=3 Suppl |pages=204S–33S |year=2004 |pmid=15383473 }}</ref> The guidelines also state "if treatment is not urgent (eg, chronic stable atrial fibrillation), warfarin administration, without concurrent heparin administration, can be commenced out-of-hospital with an anticipated maintenance dose of 4 to 5 mg per day."<ref name="pmid15383473"/> | ||
Since publication of the [[systematic review]]<ref name="pmid15522981"/> and [[clinical practice guideline]]s<ref name="pmid15383473"/>, a nonblinded, [[randomized controlled trial]] | Since publication of the [[systematic review]]<ref name="pmid15522981"/> and [[clinical practice guideline]]s<ref name="pmid15383473"/>, a nonblinded, [[randomized controlled trial]]<ref name="pmid21035980">{{cite journal| author=Farahmand S, Saeedi M, Seyed Javadi HH, Khashayar P| title=High doses of warfarin are more beneficial than its low doses in patients with deep vein thrombosis. | journal=Am J Emerg Med | year= 2011 | volume= 29 | issue= 9 | pages= 1222-6 | pmid=21035980 | doi=10.1016/j.ajem.2010.07.008 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21035980 }} </ref> found no difference between algorithms, but also achieved less frequent anticoagulation. The reason is not clear as the Kovacs 10 mg algorithm is very specific on each dose of warfarin. The last trial is also unique in that all patients were receiving a fondaparinux bridge. | ||
'''Additional algorithms:'''<br> | '''Additional algorithms:'''<br> | ||
* The Tait 5 mg regimen is for outpatient anticoagulation. Patients are given 5 mg of warfarin per day for 5 days and then the INR is checked on day 5 to determine further dosing. ([http://www.blackwell-synergy.com/action/showPopup?citid=citart1&id=f1&doi=10.1046%2Fj.1365-2141.1998.00716.x summary])<ref name="pmid9633885">{{cite journal |author=Tait RC, Sefcick A |title=A warfarin induction regimen for out-patient anticoagulation in patients with atrial fibrillation |journal=Br. J. Haematol. |volume=101 |issue=3 |pages=450-4 |year=1998 |pmid=9633885 |doi=10.1046/j.1365-2141.1998.00716.x}}</ref> | * The Tait 5 mg regimen is for outpatient anticoagulation. Patients are given 5 mg of warfarin per day for 5 days and then the INR is checked on day 5 to determine further dosing. ([http://www.blackwell-synergy.com/action/showPopup?citid=citart1&id=f1&doi=10.1046%2Fj.1365-2141.1998.00716.x summary])<ref name="pmid9633885">{{cite journal |author=Tait RC, Sefcick A |title=A warfarin induction regimen for out-patient anticoagulation in patients with atrial fibrillation |journal=Br. J. Haematol. |volume=101 |issue=3 |pages=450-4 |year=1998 |pmid=9633885 |doi=10.1046/j.1365-2141.1998.00716.x}}</ref> | ||
* The Fennerty 10 mg regimen is an older regimen that has not been studied in an [[randomized controlled trial]].<ref name="pmid6424820">{{cite journal |author=Fennerty A | * The Fennerty 10 mg regimen is an older regimen that has not been studied in an [[randomized controlled trial]].<ref name="pmid6424820">{{cite journal |author=Fennerty A ''et al.'' |title=Flexible induction dose regimen for warfarin and prediction of maintenance dose |journal=Br Med J (Clin Res Ed) |volume=288 |pages=1268–70 |year=1984 |pmid=6424820 }} [[http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=citizendium&pubmedid=6424820 PubMed Central]]</ref><ref name="pmid3144365">{{cite journal |author=Fennerty A ''et al.'' |title=Anticoagulants in venous thromboembolism |journal=BMJ |volume=297 |issue=6659 |pages=1285-8 |year=1988 |pmid=3144365}}</ref> | ||
====Pharmacogenetic guided dosing==== | ====Pharmacogenetic guided dosing==== | ||
* | Randomized controlled trials: | ||
* | |||
* A [[Pharmacogenomics|pharmacogenetic]]-guided algorithm using ''[[3CYP2C9]]'' and ''[[VKORC1]]'' outperformed an unvalidated, clinical algorithm in modeled results.<ref name="pmid19228618">{{cite journal |author=Klein TE | * In the COAG trial, [[Pharmacogenomics|pharmacogenetic]]-guided algorithm using ''[[3CYP2C9]]'' and ''[[VKORC1]]'' was compared to an algorythm that projected doses of warfarin based on clinical parameters entered into a [[clinical prediction rule]]. The study lasted 4 weeks. The proportion of time that the [[International Normalized Ratio]] was in therapeutic range was not statistically significantly different in the two groups (45.2% vs 45.4%, respectively).<ref name="pmid24251361">{{cite journal| author=Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF et al.| title=A Pharmacogenetic versus a Clinical Algorithm for Warfarin Dosing. | journal=N Engl J Med | year= 2013 | volume= | issue= | pages= | pmid=24251361 | doi=10.1056/NEJMoa1310669 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24251361 }} </ref> | ||
* A [[Pharmacogenomics|pharmacogenetic]]-based model from a cohort of orthopedic patients using ''[[3CYP2C9]]'' and ''[[VKORC1]]'' genotype results predicted 80% of the variation in warfarin doses. It is awaiting validation in larger populations and has not been reproduced in those who require warfarin for other indications.<ref>{{cite journal |author=Millican E | * In the EU-PACT trial, a [[Pharmacogenomics|pharmacogenetic]]-guided algorithm using ''[[3CYP2C9]]'' and ''[[VKORC1]]'' was compared to usual care. The study lasted 3 months. The proportion of time that the [[International Normalized Ratio]] was in therapeutic range was statistically higher in the [[Pharmacogenomics|pharmacogenetic]]-guided group (67.4% vs 60.3%), but not after week 8. <ref name="pmid24251363">{{cite journal| author=Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T et al.| title=A Randomized Trial of Genotype-Guided Dosing of Warfarin. | journal=N Engl J Med | year= 2013 | volume= | issue= | pages= | pmid=24251363 | doi=10.1056/NEJMoa1311386 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24251363 }} </ref> | ||
* In another trial, [[Pharmacogenomics|pharmacogenetic]]-guided algorithm using only ''[[CYP2C9]]'' was compared to a local algorithm. The length of the trial was not explicitly stated but may have been until stabilization of the INR. The proportion of time that the [[International Normalized Ratio]] was in therapeutic range was statistically higher in the [[Pharmacogenomics|pharmacogenetic]]-guided group (80.4% vs 63.4%, respectively). The [[Pharmacogenomics|pharmacogenetic]]-guided algorithm led to less minor bleeding (3.2 vs 12.5%, P<0.02).<ref name="pmid17851566"/> | |||
* The Kovacs 10 mg algorithm performed similarly to a [[Pharmacogenomics|pharmacogenetic]]-guided algorithm using ''[[3CYP2C9]]'' and ''[[VKORC1]]''.<ref name="pmid17989110">{{cite journal |author=Anderson JL ''et al.'' |title=Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation |journal=Circulation |volume=116|pages=2563–70 |year=2007 |pmid=17989110 }}</ref> The proportion of patients who did not have an out-of-range [[International Normalized Ratio]] statistically insignificantly 1.04 times higher in the pharmacogenetic group (69% vs 67%). | |||
Observational studies: | |||
* A [[Pharmacogenomics|pharmacogenetic]]-guided algorithm using ''[[3CYP2C9]]'' and ''[[VKORC1]]'' outperformed an unvalidated, clinical algorithm in modeled results.<ref name="pmid19228618">{{cite journal |author=Klein TE ''et al.'' |title=Estimation of the warfarin dose with clinical and pharmacogenetic data |journal=N. Engl. J. Med. |volume=360 |pages=753–64 |year=2009 |pmid=19228618 }}</ref> In this [[cohort study]] of 5052 patients, the pharmacogenetic estimated a dose within 20% of the actual dose 1.2 times more often than the clinical algorithm (46% vs 38%). | |||
* A [[Pharmacogenomics|pharmacogenetic]]-based model from a cohort of orthopedic patients using ''[[3CYP2C9]]'' and ''[[VKORC1]]'' genotype results predicted 80% of the variation in warfarin doses. It is awaiting validation in larger populations and has not been reproduced in those who require warfarin for other indications.<ref>{{cite journal |author=Millican E ''et al.'' |title=Genetic-based dosing in orthopaedic patients beginning warfarin therapy |journal= |volume=110 |issue=5 |pages=1511-5 |year=2007 |pmid=17387222 }} [http://www.warfarindosing.org Online tool based on the study].</ref> | |||
[[Cost-benefit analysis]]<ref name="pmid19153410">{{cite journal| author=Eckman MH ''et al.''| title=Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. | journal=Ann Intern Med | year= 2009 | volume= 150 | pages= 73-83 | pmid=19153410}} </ref> and [[decision analysis]]<ref name="pmid19255811">{{cite journal |author=Eckman MH, ''et al.'' |title=Should we test for CYP2C9 before initiating anticoagulant therapy in patients with atrial fibrillation? |journal=J Gen Intern Med |volume=24 |pages=543–9 |year=2009 |pmid=19255811 }}</ref> concluded that patients at average risk do not benefit from testing for CYP2C9. | |||
===Adjusting the maintenance dose=== | ===Adjusting the maintenance dose=== | ||
:See also [[atrial fibrillation]] | |||
The | The goal is [[International_Normalized_Ratio|INR]] is 2 to 3,<ref name="pmid15772203">{{cite journal| author=Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ| title=Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. | journal=Heart | year= 2005 | volume= 91 | issue= 4 | pages= 472-7| pmid=15772203 | doi=10.1136/hrt.2004.042465 | pmc=PMC1768813 |url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15772203 }} </ref> so then adjustments should be made when the INR is 1.7 or less or when 3.3 or greater.<ref name="pmid18983486">{{cite journal |author=Rose AJ, Ozonoff A, Berlowitz DR, Henault LE, Hylek EM |title=Warfarin dose management affects INR control |journal=J. Thromb. Haemost.|volume=7 |issue=1 |pages=94–101 |year=2009 |month=January |pmid=18983486 |doi=10.1111/j.1538-7836.2008.03199.x|url=http://dx.doi.org/10.1111/j.1538-7836.2008.03199.x |issn=}}</ref>A [[meta-analysis]] observed that the time in therapeutic range varies by setting:<ref name="pmid16685005">{{cite journal| author=van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ| title=Effect of study setting on anticoagulation control: a systematic review and metaregression. | journal=Chest | year= 2006 | volume= 129 | issue= 5 | pages= 1155-66 | pmid=16685005 | doi=10.1378/chest.129.5.1155 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16685005 }} </ref> | ||
* Anticoagulation clinics: 66% | |||
* Other clinics: 57% | |||
The protocol for the RELY trial<ref name="pmid19717844">{{cite journal| author=Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al.| title=Dabigatran versus warfarin in patients with atrial fibrillation. | journal=N Engl J Med | year= 2009 | volume= 361 | issue= 12 | pages= 1139-51 | pmid=19717844 | doi=10.1056/NEJMoa0905561 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19717844 }} [http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20083817 Review in: Ann Intern Med. 2010 Jan 19;152(2):JC1-2] </ref>, which yielded 64% TTR is online.<ref name="pmid19376304">{{cite journal| author=Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S et al.| title=Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. | journal=Am Heart J | year= 2009 | volume= 157 | issue= 5 | pages= 805-10, 810.e1-2 | pmid=19376304 | doi=10.1016/j.ahj.2009.02.005 | pmc= | rl=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19376304 }} </ref> Increased use of the RELY protocol is associated with increased time in thereapeutic range.<ref name="pmid23027801">{{cite journal| author=Van Spall HG, Wallentin L, Yusuf S, Eikelboom JW, Nieuwlaat R, Yang S et al.| title=Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries: an analysis of patients receiving warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. | journal=Circulation | year= 2012 | volume= 126 | issue= 19 | pages= 2309-16 | pmid=23027801 | doi=10.1161/CIRCULATIONAHA.112.101808 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23027801 }} </ref> | |||
The protocol for the CoumaGen-II, yielded 58% TTR as is [http://circ.ahajournals.org/content/125/16/1997/suppl/DC1 online].<ref name="pmid22431865">{{cite journal| author=Anderson JL, Horne BD, Stevens SM, Woller SC, Samuelson KM, Mansfield JW et al.| title=A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). | journal=Circulation | year= 2012 | volume= 125 | issue= 16 | pages= 1997-2005 | pmid=22431865 | doi=10.1161/CIRCULATIONAHA.111.070920 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22431865 }} </ref> | |||
The protocol for the ARISTOTLE trial, yielded 62% TTR.<ref name="pmid21870978">{{cite journal| author=Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M et al.| title=Apixaban versus warfarin in patients with atrial fibrillation. | journal=N Engl J Med | year= 2011 | volume= 365 | issue= 11 | pages= 981-92 | pmid=21870978 | doi=10.1056/NEJMoa1107039 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21870978 }} [http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22250164 Review in: Ann Intern Med. 2012 Jan 17;156(2):JC1-2, JC1-3] </ref><ref name="pmid20211292">{{cite journal| author=Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD et al.| title=Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. | journal=Am Heart J | year= 2010 | volume= 159 | issue= 3 | pages= 331-9 | pmid=20211292 | doi=10.1016/j.ahj.2009.07.035 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20211292 }} </ref> | |||
The optimal [[International_Normalized_Ratio|INR]]is unclear.<ref name="pmid19597069">{{cite journal |author=Torn M ''et al.'' |title=Optimal level of oral anticoagulant therapy for the prevention of arterial thrombosis in patients with mechanical heart valve prostheses, atrial fibrillation, or myocardial infarction: a prospective study of 4202 patients |journal=Arch Intern Med |volume=169 |pages=1203–9 |year=2009 |month=July |pmid=19597069 }}</ref> | |||
While usually warfarin is monitored by measing the [[International_Normalized_Ratio|INR]] once a month at a [[healthcare provider]]'s office, other alternatives are: | |||
* Some patients may use home monitoring.<ref>{{Cite journal | issn = 0140-6736 | last = Heneghan | first = Carl | coauthors = Alison Ward, Rafael Perera | title = Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data | |||
| journal = The Lancet | date = 2011-12-01 |doi:10.1016/S0140-6736(11)61294-4}}</ref> | |||
* Patients whose dose is stable for at least 6 months, may not require monthly monitoring.<ref name="pmid22084331">{{cite journal| author=Schulman S, Parpia S, Stewart C, Rudd-Scott L, Julian JA, Levine M| title=Warfarin dose assessment every 4 weeks versus every 12 weeks in patients with stable international normalized ratios: a randomized trial. | journal=Ann Intern Med | year= 2011 | volume= 155 | issue= 10 | pages= 653-9 | pmid=22084331 | doi=10.1059/0003-4819-155-10-201111150-00003 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22084331 }} </ref> | |||
===Discontinuation before procedures=== | ===Discontinuation before procedures=== | ||
Management of warfarin around invasive procedures has been reviewed<ref name="pmid9154771">{{cite journal |author=Kearon C, Hirsh J |title=Management of anticoagulation before and after elective surgery |journal=N | Details have been provided by the [[American College of Chest Physicians]] at [http://chestjournal.chestpubs.org/content/133/6_suppl/299S.full The Perioperative Management of Antithrombotic Therapy]. | ||
Management of warfarin around invasive procedures has been reviewed<ref name="pmid9154771">{{cite journal |author=Kearon C, Hirsh J |title=Management of anticoagulation before and after elective surgery |journal=N Engl J Med |volume=336|pages=1506–11 |year=1997|pmid=9154771}}</ref> and new bridges have been studied.<ref name="pmid19470892">{{cite journal |author=Pengo V, Cucchini U, Denas G, ''et al.'' |title=Standardized low-molecular-weight heparin bridging regimen in outpatients on oral anticoagulants undergoing invasive procedure or surgery: an inception cohort management study |journal=Circulation |volume=119 |pages=2920–7 |year=2009 |pmid=19470892}}</ref> | |||
For procedures that need discontinuation of [[anticoagulation]], a cohort study that found that interruption for 5 days or less was generally safe.<ref name="pmid18195197">{{cite journal |author=Garcia DA ''et al.'' |title=Risk of thromboembolism with short-term interruption of warfarin therapy |journal=Arch Intern Med|volume=168 |pages=63–9 |year=2008 |pmid=18195197}}</ref> | |||
For patients needing a bridge, low molecular weight heparin is preferable to [[intravenous infusion]] of unfractionated heparin.<ref name="pmid18574269">{{cite journal| author=Douketis JD ''et al.''| title=The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) | journal=Chest | year= 2008 | volume= 133 | issue= 6 Suppl | pages= 299S-339S | pmid=18574269 | |||
}} </ref> For patients needing a low molecular weight heparin bridge, a [http://archinte.ama-assn.org/cgi/content/full/164/12/1319/TABLEIOI30313T1 protocol] is available.<ref name="pmid15226166">{{cite journal |author=Douketis JD, Johnson JA, Turpie AG |title=Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen |journal=Arch Intern Med|volume=164 |pages=1319–26 |year=2004 |month=June |pmid=15226166 }}</ref> One bridge before coronary artery bypass grafting is to stop 6 days before and give 5 mg/day of vitamin K on the day of warfarin cessation and use low molecular weight [[heparin]] with last dose the night before surgery.<ref name="pmid17588394">{{cite journal |author=Whitlock RP ''et al.'' |title=Warfarin cessation before cardiopulmonary bypass: lessons learned from a randomized controlled trial of oral vitamin K |journal=Ann Thorac Surg |volume=84 |issue=1 |pages=103–8 |year=2007 |pmid=17588394}}</ref>However, bridging with low molecular weight heparin increases bleeding as compared to no bridging.<ref name="pmid19719825">{{cite journal| author=Witt DM ''et al.''| title=Incidence and predictors of bleeding or thrombosis after polypectomy in patients receiving and not receiving anticoagulation therapy. | journal=J Thromb Haemost | year= 2009 | volume= 7| pages= 1982-9 | pmid=19719825}} </ref> | |||
==Effectiveness== | |||
The effect of warfarin is measured by the [[prothrombin time]] (or the [[International Normalized Ratio]] derived from the prothrombin time) although warfarin can also affect the partial thromboplastin time.<ref name="pmid3397749">{{cite journal |author=Bell DF ''et al.'' |title=Elevated partial thromboplastin time as an indicator of hemorrhagic risk in postoperative patients on warfarin prophylaxis |journal=J Arthroplasty |volume=3 |pages=181–4 |year=1988 |pmid=3397749 }}</ref><ref name="pmid3816546">{{cite journal |author=Hauser VM, Rozek SL |title=Effect of warfarin on the activated partial thromboplastin time |journal=Drug Intell Clin Pharm |volume=20 |pages=964–7 |year=1986 |pmid=3816546 }}</ref> | |||
Warfarin's effectiveness depends the proportion of time that a patient's spends in the therapeutic range of anticoagulation, time in the optimum therapeutic range - the time in therapeutic range (TTR). In carefully conducted and monitored randomized controlled trials, the TTR ranges from 55% to 65%.<ref>{{cite web |url= http://www.nejm.org/doi/full/10.1056/NEJMe1109748?query=featured_home |title=A New Era for Anticoagulation in Atrial Fibrillation — NEJM |first=Jessica |last=Mega |work=nejm.org |year=2011 [last update] |accessdate=August 29, 2011}}</ref> However, the TTR is lower in the community practice.<ref name="pmid10761962">{{cite journal| author=Samsa GP, Matchar DB, Goldstein LB, Bonito AJ, Lux LJ, Witter DM et al.| title=Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. | journal=Arch Intern Med | year= 2000 | volume= 160 | issue= 7 | pages= 967-73 | pmid=10761962 | doi= | pmc= | url= }} </ref><ref name="pmid16685005">{{cite journal| author=van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ| title=Effect of study setting on anticoagulation control: a systematic review and metaregression. | journal=Chest | year= 2006 | volume= 129 | issue= 5 | pages= 1155-66 | pmid=16685005 | doi=10.1378/chest.129.5.1155 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16685005 }} </ref><ref name="pmid17260164">{{cite journal| author=McBride D, Brüggenjürgen B, Roll S, Willich SN| title=Anticoagulation treatment for the reduction of stroke in atrial fibrillation: a cohort study to examine the gap between guidelines and routine medical practice. | journal=J Thromb Thrombolysis | year= 2007 | volume= 24 | issue= 1 | pages= 65-72 | pmid=17260164 | doi=10.1007/s11239-006-0002-8 | pmc= | url= }} </ref><ref name="pmid16475046">{{cite journal| author=Pengo V, Pegoraro C, Cucchini U, Iliceto S| title=Worldwide management of oral anticoagulant therapy: the ISAM study. | journal=J Thromb Thrombolysis | year= 2006 | volume= 21 | issue= 1 | pages= 73-7 | pmid=16475046 | doi=10.1007/s11239-006-5580-y | pmc= | url= }} </ref> The TTR may be calculated with linear interpolation between INR values<ref name="pmid16475046"/>, or may be more simply reported as the number of INR values out of range<ref name="pmid17260164"/>. The highest reported TTR is approximately 75%.<ref name="pmid21616951">{{cite journal| author=Wieloch M, Själander A, Frykman V, Rosenqvist M, Eriksson N, Svensson PJ| title=Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. | journal=Eur Heart J | year= 2011 | volume= 32 | issue= 18 | pages= 2282-9 | pmid=21616951 | doi=10.1093/eurheartj/ehr134 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21616951 }} </ref> | |||
==Interactions and contraindications== | ==Interactions and contraindications== | ||
Warfarin interacts with many medications. A proposed classifications of mechanisms is:<ref name="pmid17698826">{{cite journal |author=Juurlink DN |title=Drug interactions with warfarin: what clinicians need to know |journal=CMAJ : Canadian Medical Association journal &#61; journal de l'Association medicale canadienne |volume=177 | Warfarin interacts with many medications. A proposed classifications of mechanisms is:<ref name="pmid17698826">{{cite journal |author=Juurlink DN |title=Drug interactions with warfarin: what clinicians need to know |journal=CMAJ : Canadian Medical Association journal &#61; journal de l'Association medicale canadienne |volume=177|pages=369–71 |year=2007 |pmid=17698826 }}</ref> | ||
* Interference with platelet function | * Interference with platelet function | ||
* Injury to gastrointestinal mucosa | * Injury to gastrointestinal mucosa | ||
| Line 127: | Line 170: | ||
* Interruption of the vitamin K cycle | * Interruption of the vitamin K cycle | ||
Some foodstuffs have also been reported to interact with warfarin.<ref name="pmid15911722">{{cite journal |author=Holbrook AM | Some foodstuffs have also been reported to interact with warfarin.<ref name="pmid15911722">{{cite journal |author=Holbrook AM ''et al.'' |title=Systematic overview of warfarin and its drug and food interactions |journal=Arch. Intern Med. |volume=165 |pages=1095–106 |year=2005 |pmid=15911722 }}</ref> | ||
==Adverse effects== | ==Adverse effects== | ||
The most accurate clinical prediction rule for estimating the risk of bleeding is the [http://www.mdcalc.com/has-bled-score-for-major-bleeding-risk/ HAS-BLED] score.<ref>Lopes RD, Crowley MJ, Shah BR, et al . Stroke Prevention in Atrial Fibrillation. Comparative | |||
Effectiveness Review No. 123. AHRQ Publication No. 13-EHC113-EF. Rockville, MD: Agency for Healthcare Research and Quality; August 2013. http://www.effectivehealthcare.ahrq.gov/ reports/final.cfm.</ref> | |||
The risk of bleeding can be predicted with the ATRIA score based on:<ref name="pmid21757117">{{cite journal| author=Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N et al.| title=A New Risk Scheme to Predict Warfarin-Associated Hemorrhage The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. | journal=J Am Coll Cardiol | year= 2011 | volume= 58 | issue= 4 | pages= 395-401 | pmid=21757117 | doi=10.1016/j.jacc.2011.03.031 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21757117 }} </ref> | |||
* [[anemia]] (3 points) | |||
* [[glomerular filtration rate]] <30 ml/min or receiving dialysis | |||
* age 75 years or more (2 points) | |||
* prior bleeding (1 point) | |||
* [[hypertension]] (1 point) | |||
The annual risk of bleeding was: | |||
* Low risk (0 to 3 points) - 0.8% | |||
* Intermediate risk (4 points) - 2.6% | |||
* High risk (5 to 10 points) - 5.8% | |||
===Elderly patients=== | ===Elderly patients=== | ||
Patients aged 80 years or more may be especially susceptible to bleeding complications with a rate of 13 bleeds per 100 person-years.<ref name="pmid17515465">{{cite journal |author=Hylek EM | Patients aged 80 years or more may be especially susceptible to bleeding complications with a rate of 13 bleeds per 100 person-years.<ref name="pmid17515465">{{cite journal |author=Hylek EM ''et al.'' |title=Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation |journal=Circulation |volume=115 |pages=2689-96 |year=2007 |pmid=17515465}}</ref> | ||
===Patients with prior intracranial hemorrhage from warfarin=== | ===Patients with prior intracranial hemorrhage from warfarin=== | ||
These patients are at high risk of bad outcomes regardless of whether anticoagulation is resumed.<ref name="pmid18852344">{{cite journal |author=Claassen DO | These patients are at high risk of bad outcomes regardless of whether anticoagulation is resumed.<ref name="pmid18852344">{{cite journal |author=Claassen DO ''et al.'' |title=Restarting anticoagulation therapy after warfarin-associated intracerebral hemorrhage |journal=Arch Neurol |volume=65 |pages=1313–8 |year=2008 |pmid=18852344 }}</ref> | ||
===Patients with cancer=== | ===Patients with cancer=== | ||
| Line 159: | Line 214: | ||
===Unstable anticoagualation=== | ===Unstable anticoagualation=== | ||
Supplementing warfarin with 150 micrograms of [[vitamin k]] may reduce the frequency of unstable anticoaguation among patients who are difficult to anticoagulate.<ref name="pmid17110451">{{cite journal |author=Sconce E | Supplementing warfarin with 150 micrograms of [[vitamin k]] may reduce the frequency of unstable anticoaguation among patients who are difficult to anticoagulate.<ref name="pmid17110451">{{cite journal |author=Sconce E ''et al.'' |title=Vitamin K supplementation can improve stability of anticoagulation for patients with unexplained variability in response to warfarin |journal=Blood |volume=109 |pages=2419–23 |year=2007 |pmid=17110451 }}</ref> This amount of vitamin k led to 16% increase in the warfarin dose from 3.8 mg per day to 4.4 mg per day after one week. | ||
===Antagonism and reversal=== | ===Antagonism and reversal=== | ||
A [http:// | A [http://chestjournal.chestpubs.org/content/133/6_suppl/160S/T5.expansion.html detailed table] on reversing warfarin are provided in [[clinical practice guideline]]s from the [[American College of Chest Physicians]].<ref name="pmid18574265"/> | ||
For patients who are not bleeding and have an [[International Normalized Ratio]] (INR) between 4.5 and 10.0, either withholding warfarin<ref>1{{Cite journal | For patients who are not bleeding and have an [[International Normalized Ratio]] (INR) between 4.5 and 10.0, either withholding warfarin<ref>1{{Cite journal | ||
| volume = 150 | | volume = 150 | ||
| pages = 293-300 | | pages = 293-300 | ||
| last = Crowther | | last = Crowther | ||
| first = | | first = MA | ||
| coauthors = | | coauthors = ''et al.'' | ||
| title = Oral Vitamin K Versus Placebo to Correct Excessive Anticoagulation in Patients Receiving Warfarin: A Randomized Trial | | title = Oral Vitamin K Versus Placebo to Correct Excessive Anticoagulation in Patients Receiving Warfarin: A Randomized Trial | ||
| journal = Ann Intern Med | | journal = Ann Intern Med | ||
| date = 2009-03-03 | | date = 2009-03-03 | ||
| url = http://www.annals.org/cgi/content/abstract/150/5/293 | | url = http://www.annals.org/cgi/content/abstract/150/5/293 | ||
}}</ref> or 1 mg of oral vitamin K is effective<ref name="pmid12186515">{{cite journal |author=Crowther MA | }}</ref> or 1 mg of oral vitamin K is effective<ref name="pmid12186515">{{cite journal |author=Crowther MA ''et al.'' |title=Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial |journal=Ann Intern Med |volume=137 |pages=251-4 |year=2002 |pmid=12186515 }}</ref>. Vitamin K should not be given subcutaneously, even at low doses then can lower the INR too much.<ref name="pmid16505257">{{cite journal |author=Dezee KJ ''et al.'' |title=Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis |journal=Arch Intern Med |volume=166 |pages=391–7 |year=2006 |pmid=16505257}}</ref> | ||
==Drug interactions== | |||
===Acetaminophen=== | |||
Acetaminophen in doses of 2 grams per day or more for several consecutive days may interact with warfarin.<ref>Hughes GJ, Patel PN, Saxena N.. Effect of Acetaminophen on International Normalized Ratio in Patients Receiving Warfarin Therapy. Pharmacotherapy 2011 June;31(6):591–597. </ref><ref name="pmid21191575">{{cite journal| author=Zhang Q ''et al.''| title=Interaction between acetaminophen and warfarin in adults receiving long-term oral anticoagulants: a randomized controlled trial. | journal=Eur J Clin Pharmacol | year= 2011 | volume= 67| pages= 309-14 | pmid=21191575 }} </ref> | |||
===Anti-platelet agents=== | |||
Patients taking aspirin, clopidogrel, or dipyridamole may be at higher risk of hemorrhage.<ref name="pmid18198244">{{cite journal |author=Johnson SG ''et al.'' |title=Outcomes associated with combined antiplatelet and anticoagulant therapy |journal=Chest |volume=133 |issue=4 |pages=948–54 |year=2008 |pmid=18198244 }}</ref> | |||
===Antilipemic agents=== | |||
Medications, such as the [[antilipemic agent]]s [[atorvastatin]], [[simvastatin]], or [[gemfibrozil]] that are metabolized by [[cytochrome P-450]] CYP3A4 may increase bleeding when added to patients taking warfarin.<ref name="pmid20103024">{{cite journal| author=Schelleman H ''et al.'' | title=Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk. | journal=Am J Med | year= 2010 | volume= 123| pages= 151-7 | pmid=20103024 }}</ref> | |||
===Antibiotics=== | |||
Antibiotics metabolized by [[cytochrome P-450]] CYP2C9, such as [[trimethoprim-sulfamethoxazole]] and [[ciprofloxacin]] may increase risk of [[hospitalization]] for [[gastrointestinal hemorrhage]].<ref>{{Cite journal | doi = 10.1001/archinternmed.2010.37 | volume = 170 | pages = 617-621 | last = Fischer | first = HD | coauthors = ''et al.'' | title = Hemorrhage during warfarin therapy associated with cotrimoxazole and other urinary tract anti-infective agents: a population-based study | journal = Arch Intern Med | date = 2010-04-12 | url = http://archinte.ama-assn.org/cgi/content/abstract/170/7/617 }}</ref> | |||
==References== | ==References== | ||
<references/> | <small> | ||
<references> | |||
</references> | |||
</small> | |||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 17:00, 6 November 2024
Warfarin (IUPAC name 4-hydroxy-3-(3-oxo-1-phenylbutyl)-2H-chromen-2-one), also widely called coumadin, is an anticoagulant medication used prophylactically to suppress the formation of embolism and thrombosis from conditions such as atrial fibrillation, deep venous thrombosis, and pulmonary embolism.
Originally designed to be a rat poison, warfarin works as an anticoagulant by suppressing the enzyme epoxide reductase in the liver, thereby suppressing the formation of the reduced form of vitamin K epoxide, which is needed for the synthesis of many coagulation factors. As a drug, it is often sold as the sodium salt of warfarin.
Discovery
The dangers of feeding livestock spoiled sweet clover hay were known in the 1920’s[1] and scientists at the University of Wisconsin-Madison were spearheading the problem on multiple fronts. R.A. Brink and W.K. Smith were attempting to breed a new variety of clover that was free of the toxic effect and Karl Paul Link's laboratory was attempting to isolate the killer compound. Farmers were already hurting due to the Great Depression and despite knowing they should not feed their livestock such hay, they could not afford to buy uncontaminated supplies. This disease that was affecting many livestock throughout the US became known as "sweet clover disease".[2]
The urgency of this work was punctuated in the winter of 1933 when Ed Carlson, a farmer from Deer Park, Wisconsin, came to Madison for help carrying a milk can of uncoagulated blood, a dead cow in his truck and a sample of the hay contaminated with the spoiled sweet clover. This spurred Link on and in 1940 he finally published the source of the hemorrhagic factor in sweet clover, 3,3'-methylene-bis[4-hyfroxycoumarin], known as coumarin.[3] The following year Link published the discovery of dicumarol, the oxidised form of coumarin that was present in the spoiled sweet clover; this became widely used as an oral anticoagulants for medical treatment.[4][5]
Given the death of cattle from hemorrhaging, Link began to realise that anticoagulants had potential to be rodenticides.
I had an intuitive feeling that this might be a good thing. A pretty bad thing for rats, but a good thing for humans. But the idea didn't come overnight. It came into my head and the heads of everyone in the lab over a period of years. [6]
Dicumarol turned out to be a poor rodenticide as it acted too slowly[7] but by 1948 Link had patented another coumarin derivative as a rodenticide. Research for both dicumarol and the new rodenticide was funded by the Wisconsin Alumni Research Foundation (WARF) and the brand name for these anticoagulating rat posions was coined 'Warfarin' after WARF. Later, the rodenticide was promoted for clinical applications under the brand name 'Coumadin'.
Mechanism of action
Warfarin therapy reduces the vitamin K dependent cofactors II, VII, IX, and X and the vitamin K dependent Protein C. The level of factor II is thought to most influence coagulation.[8][9] The levels of factor VII and Protein C fall the fastest after warfarin is started.[9] With the exception of Factor IX, these factors are from either the extrinsic pathway or the final common pathway.
Pharmacokinetics
Absorption
Distribution
Metabolism
Pharmacogenomics
About 15% of the effect of warfarin can be explained by the amount of warfarin in the blood.[10] About 50% of the effect of warfarin can be explained by genetic factors.[11][12][13] The American Food and Drug Administration "highlights the opportunity for healthcare providers to use genetic tests to improve their initial estimate of what is a reasonable warfarin dose for individual patients".[14] A systematic review concluded that as of early 2009, there is insufficient benefit in genetic testing.[15]
VKORC1
Genetic polymorphisms in the vitamin K epoxide reductase complex 1 (VKORC1) gene explain 30% of the dose variation between patients[16]: particular mutations make VKORC1 less susceptible to suppression by warfarin[17] There are a main haplotypes that explain 25% of variation: low-dose haplotype group (A) and a high-dose haplotype group (B).[18] For the three combinations of the haplotypes, the mean daily maintenance dose of warfarin was:
- A/A: 2.7+/-0.2 mg
- A/B: 4.9+/-0.2 mg
- B/B: 6.2+/-0.3 mg
VKORC1 polymorphisms also explain why African Americans are relatively resistant to warfarin (higher proportion of group B haplotypes), while Asian Americans are more sensitive (higher proportion of group A haplotypes).[18]
CYP2C9
CYP2C9 is an isoenzyme of cytochrome P-450. Polymorphisms of CYP2C9 explain another 10% of variation in warfarin dosing[16], mainly among Caucasian patients as these variants are rare in African American and most Asian populations.[19] A meta-analysis of mainly Caucasian patients found[19]:
- CYP2C9*2 allele:
- present in 12.2% of patients
- mean reduction was in warfarin dose was 0.85 mg (17% reduction)
- relative bleeding risk was 1.91
- CYP2C9*3 allele:
- present in 7.9% of patients
- mean reduction was in warfarin dose was 1.92 mg (37% reduction)
- relative bleeding risk was 1.77
Excretion
Availability
Generic preparations have similar pharmacology.[20]
Dosage
Clinical practice guidelines are available for using warfarin.[21]
"After 4 to 5 days of concomitant warfarin and heparin therapy, heparin is discontinued when the INR has been in the therapeutic range on two measurements approximately 24 h apart."[22]
Loading regimens
A decision analysis "suggests that patients with intermediate and high/likely probabilities of PE benefit from preemptive anticoagulation". [23]
Because of warfarin's difficult pharmacokinetics, researchers have proposed algorithms for warfarin loading.
| Dosing | Study | Time till a therapeutic INR (days) |
Rate of anticoagulation (INR=2-3) |
Rate of over-coagulation | |
|---|---|---|---|---|---|
| Kovacs 10 mg | 10 mg/day for two days then specified adjusted dosing |
Kovacs†[24] | 4.2 | 83% by day 5 | 9% within 4 weeks(INR>5) (most occurred after day 10) |
| Quiroz‡[26] Mean age: 51 United States |
5 (two consecutive INRs≥2) |
56% at 5 days | 0% within 5 days (INR>5) | ||
| Anderson‡[27] | 69% at 5 days | ||||
| Farahmand‡[28] Mean age: 48 Iran |
27% at 5 days | 2% at 5 days (INR>5) | |||
| Harrison 10 mg | 10 mg/day for one day then flexible adjusted dosing |
Harrison¶[9] | 63% at 3.5 days | 17% within 3.5 days (INR>4.8) | |
| Crowther¶[25] | 69% at 5 days | 0% within 5 days (INR>5) 24% within 5 days (INR>3) | |||
| Kovacs 5 mg | 5 mg/day for two days then specified adjusted dosing |
Kovacs†[24] | 5.6 | 46% by day 5 | 11% within 4 weeks (INR>5) (most occurred after day 10) |
| Farahmand‡[28] Mean age: 48 Iran |
47% at 5 days | 2% at 5 days (INR>5) | |||
| Harrison 5 mg | 5 mg/day for one day then flexible adjusted dosing |
Harrison¶ [9] | 80% at 3.5 days | 4% within 4.5 days (INR>4.8) | |
| Crowther¶[25] | 88% at 5 days | 3% within 5 days (INR>5) 7% within 5 days (INR>3) | |||
| Quiroz‡[26] Mean age: 51 United States |
5 (two consecutive INRs≥2) |
52% at 5 days | 0% within 5 days (INR>5) | ||
| Anderson pharmacogenetic-guided algorithm | Based on CYP2C9 and VKORC1 | Anderson†[27] | 70% at 5 days | ||
| Caraco pharmacogenetic-guided algorithm | Based on CYP2C9 | Caraco[29] | 4.8 | ||
| † Blinded study. ‡ Independent study not from original investigators. ¶ Same research group (Hamilton, Ontario). Notes:
| |||||
Empiric dosing
On first look, the evidence table suggests that the Harrison 5 mg algorithm from Hamilton is the chest combination of efficacy and safety; however, two independent studies (Kovacs[24] and Quiroz in 2006 from the Massachusetts General Hospital[26]) have not been able to replicate the results of Hamilton group of Harrison and Crowther. One explanation may be that the personnel in the Harrison study were more expert and the flexibility in the algorithm allowed expression of their expertise. If so, then perhaps equally expert health care providers should use the Harrison 5 mg algorithm while other personnel should use the Kovacs 10 mg algorithm. Considering that the expertise of the Massachusetts General Hospital could not replicate the Hamilton results, perhaps most providers should use the Kovacs 10 mg algorithm, at least for inpatients.
A systematic review of the randomized controlled trials done through 2003 of 5 mg versus the 10 mg concluded that the Kovacs 10 mg regimen is best.[8] This conclusion was largely based on the inability of the results of the Harrison 5 mg flexible algorithm to be replicated by Kovacs.
Clinical practice guidelines in 2004 by the American College of Chest Physicians concluded that either 5 or 10 loads are acceptable.[30] The guidelines also state "if treatment is not urgent (eg, chronic stable atrial fibrillation), warfarin administration, without concurrent heparin administration, can be commenced out-of-hospital with an anticipated maintenance dose of 4 to 5 mg per day."[30]
Since publication of the systematic review[8] and clinical practice guidelines[30], a nonblinded, randomized controlled trial[28] found no difference between algorithms, but also achieved less frequent anticoagulation. The reason is not clear as the Kovacs 10 mg algorithm is very specific on each dose of warfarin. The last trial is also unique in that all patients were receiving a fondaparinux bridge.
Additional algorithms:
- The Tait 5 mg regimen is for outpatient anticoagulation. Patients are given 5 mg of warfarin per day for 5 days and then the INR is checked on day 5 to determine further dosing. (summary)[31]
- The Fennerty 10 mg regimen is an older regimen that has not been studied in an randomized controlled trial.[32][33]
Pharmacogenetic guided dosing
Randomized controlled trials:
- In the COAG trial, pharmacogenetic-guided algorithm using 3CYP2C9 and VKORC1 was compared to an algorythm that projected doses of warfarin based on clinical parameters entered into a clinical prediction rule. The study lasted 4 weeks. The proportion of time that the International Normalized Ratio was in therapeutic range was not statistically significantly different in the two groups (45.2% vs 45.4%, respectively).[34]
- In the EU-PACT trial, a pharmacogenetic-guided algorithm using 3CYP2C9 and VKORC1 was compared to usual care. The study lasted 3 months. The proportion of time that the International Normalized Ratio was in therapeutic range was statistically higher in the pharmacogenetic-guided group (67.4% vs 60.3%), but not after week 8. [35]
- In another trial, pharmacogenetic-guided algorithm using only CYP2C9 was compared to a local algorithm. The length of the trial was not explicitly stated but may have been until stabilization of the INR. The proportion of time that the International Normalized Ratio was in therapeutic range was statistically higher in the pharmacogenetic-guided group (80.4% vs 63.4%, respectively). The pharmacogenetic-guided algorithm led to less minor bleeding (3.2 vs 12.5%, P<0.02).[29]
- The Kovacs 10 mg algorithm performed similarly to a pharmacogenetic-guided algorithm using 3CYP2C9 and VKORC1.[27] The proportion of patients who did not have an out-of-range International Normalized Ratio statistically insignificantly 1.04 times higher in the pharmacogenetic group (69% vs 67%).
Observational studies:
- A pharmacogenetic-guided algorithm using 3CYP2C9 and VKORC1 outperformed an unvalidated, clinical algorithm in modeled results.[36] In this cohort study of 5052 patients, the pharmacogenetic estimated a dose within 20% of the actual dose 1.2 times more often than the clinical algorithm (46% vs 38%).
- A pharmacogenetic-based model from a cohort of orthopedic patients using 3CYP2C9 and VKORC1 genotype results predicted 80% of the variation in warfarin doses. It is awaiting validation in larger populations and has not been reproduced in those who require warfarin for other indications.[37]
Cost-benefit analysis[38] and decision analysis[39] concluded that patients at average risk do not benefit from testing for CYP2C9.
Adjusting the maintenance dose
- See also atrial fibrillation
The goal is INR is 2 to 3,[40] so then adjustments should be made when the INR is 1.7 or less or when 3.3 or greater.[41]A meta-analysis observed that the time in therapeutic range varies by setting:[42]
- Anticoagulation clinics: 66%
- Other clinics: 57%
The protocol for the RELY trial[43], which yielded 64% TTR is online.[44] Increased use of the RELY protocol is associated with increased time in thereapeutic range.[45]
The protocol for the CoumaGen-II, yielded 58% TTR as is online.[46]
The protocol for the ARISTOTLE trial, yielded 62% TTR.[47][48]
The optimal INRis unclear.[49]
While usually warfarin is monitored by measing the INR once a month at a healthcare provider's office, other alternatives are:
- Some patients may use home monitoring.[50]
- Patients whose dose is stable for at least 6 months, may not require monthly monitoring.[51]
Discontinuation before procedures
Details have been provided by the American College of Chest Physicians at The Perioperative Management of Antithrombotic Therapy.
Management of warfarin around invasive procedures has been reviewed[52] and new bridges have been studied.[53]
For procedures that need discontinuation of anticoagulation, a cohort study that found that interruption for 5 days or less was generally safe.[54]
For patients needing a bridge, low molecular weight heparin is preferable to intravenous infusion of unfractionated heparin.[55] For patients needing a low molecular weight heparin bridge, a protocol is available.[56] One bridge before coronary artery bypass grafting is to stop 6 days before and give 5 mg/day of vitamin K on the day of warfarin cessation and use low molecular weight heparin with last dose the night before surgery.[57]However, bridging with low molecular weight heparin increases bleeding as compared to no bridging.[58]
Effectiveness
The effect of warfarin is measured by the prothrombin time (or the International Normalized Ratio derived from the prothrombin time) although warfarin can also affect the partial thromboplastin time.[59][60]
Warfarin's effectiveness depends the proportion of time that a patient's spends in the therapeutic range of anticoagulation, time in the optimum therapeutic range - the time in therapeutic range (TTR). In carefully conducted and monitored randomized controlled trials, the TTR ranges from 55% to 65%.[61] However, the TTR is lower in the community practice.[62][42][63][64] The TTR may be calculated with linear interpolation between INR values[64], or may be more simply reported as the number of INR values out of range[63]. The highest reported TTR is approximately 75%.[65]
Interactions and contraindications
Warfarin interacts with many medications. A proposed classifications of mechanisms is:[66]
- Interference with platelet function
- Injury to gastrointestinal mucosa
- Reduced synthesis of vitamin K by intestinal flora
- Interference with warfarin metabolism by cytochrome P-450 CYP2C9 isoenzyme.
- Interruption of the vitamin K cycle
Some foodstuffs have also been reported to interact with warfarin.[67]
Adverse effects
The most accurate clinical prediction rule for estimating the risk of bleeding is the HAS-BLED score.[68]
The risk of bleeding can be predicted with the ATRIA score based on:[69]
- anemia (3 points)
- glomerular filtration rate <30 ml/min or receiving dialysis
- age 75 years or more (2 points)
- prior bleeding (1 point)
- hypertension (1 point)
The annual risk of bleeding was:
- Low risk (0 to 3 points) - 0.8%
- Intermediate risk (4 points) - 2.6%
- High risk (5 to 10 points) - 5.8%
Elderly patients
Patients aged 80 years or more may be especially susceptible to bleeding complications with a rate of 13 bleeds per 100 person-years.[70]
Patients with prior intracranial hemorrhage from warfarin
These patients are at high risk of bad outcomes regardless of whether anticoagulation is resumed.[71]
Patients with cancer
| No cancer | Cancer | ||||
|---|---|---|---|---|---|
| Stage I or II | Stage III | Stage IV | |||
| Major bleeding |
Events per 100 patient-years |
8.6 | 3.4 | 19.1 | 42.8 |
| Hazard ratio | 1 | 0.5 | 2.15 | 4.8 | |
| Recurrent VTE |
Events per 100 patient-years |
12.8 | 14.5 | 44.1 | 54.1 |
| Hazard ratio | 1 | 1.9 | 5.3 | 4.6 | |
| Adapted from Table 3 of Prandoni et al.[72] | |||||
Patients with cancer are more likely to have bleeding complications, especially if they have Stage III (regionally extensive) or IV (metastatic) cancer.[72] Regardless of the extent of cancer, the risk of bleeding was less than the risk of recurrent embolism and thromboembolism:
Unstable anticoagualation
Supplementing warfarin with 150 micrograms of vitamin k may reduce the frequency of unstable anticoaguation among patients who are difficult to anticoagulate.[73] This amount of vitamin k led to 16% increase in the warfarin dose from 3.8 mg per day to 4.4 mg per day after one week.
Antagonism and reversal
A detailed table on reversing warfarin are provided in clinical practice guidelines from the American College of Chest Physicians.[22] For patients who are not bleeding and have an International Normalized Ratio (INR) between 4.5 and 10.0, either withholding warfarin[74] or 1 mg of oral vitamin K is effective[75]. Vitamin K should not be given subcutaneously, even at low doses then can lower the INR too much.[76]
Drug interactions
Acetaminophen
Acetaminophen in doses of 2 grams per day or more for several consecutive days may interact with warfarin.[77][78]
Anti-platelet agents
Patients taking aspirin, clopidogrel, or dipyridamole may be at higher risk of hemorrhage.[79]
Antilipemic agents
Medications, such as the antilipemic agents atorvastatin, simvastatin, or gemfibrozil that are metabolized by cytochrome P-450 CYP3A4 may increase bleeding when added to patients taking warfarin.[80]
Antibiotics
Antibiotics metabolized by cytochrome P-450 CYP2C9, such as trimethoprim-sulfamethoxazole and ciprofloxacin may increase risk of hospitalization for gastrointestinal hemorrhage.[81]
References
- ↑ Wardrop D, Keeling D (2008) The story of the discovery of heparin and warfarin Brit J Haematol 141:757-63
- ↑ Duxbury BM, Poller L (2001) The oral anticoagulant saga: past, present, and future Clin Appl Thrombosis/Hemostasis 7:269–75 PMID 11697707
- ↑ Campbell HA et al. (1940) Studies on the hemorrhagic sweet clover disease. I. The preparation of hemorrhagic concentrates. J Biol Chem, 136, 47–55.
- ↑ Campbell HA et al. (1941) Studies on the hemorrhagic sweet clover disease. II. The bioassay of hemorrhagic concentrates by following the prothrombin level in the plasma of rabbit blood. J Biol Chem 138:1–20
- ↑ Stahmann MA et al. (1941) Studies on the hemorrhagic sweet clover disease. V. Identification and synthesis of the hemorrhagic agent J Biol Chem 138:513–27
- ↑ Karl Paul Link, Societal Contributions hosted by Wisconsin Alumni Research Foundation
- ↑ Last JA (2002) The missing link: the story of Karl Paul Link. Toxicol Sci 66:4–6.
- ↑ 8.0 8.1 8.2 8.3 Eckhoff CD, Didomenico RJ, Shapiro NL (2004). "Initiating warfarin therapy: 5 mg versus 10 mg". Ann Pharmacother 38: 2115–21. DOI:10.1345/aph.1E083. PMID 15522981. Research Blogging.
- ↑ 9.0 9.1 9.2 9.3 9.4 Harrison L et al. (1997). "Comparison of 5-mg and 10-mg loading doses in initiation of warfarin therapy". Ann Intern Med 126: 133–6. PMID 9005747. [e]
- ↑ White RH, Zhou H, Romano P, Mungall D (1995). "Changes in plasma warfarin levels and variations in steady-state prothrombin times.". Clin Pharmacol Ther 58 (5): 588-93. DOI:10.1016/0009-9236(95)90179-5. PMID 7586953. Research Blogging.
- ↑ Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM et al. (2008). "Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin.". Clin Pharmacol Ther 84 (3): 326-31. DOI:10.1038/clpt.2008.10. PMID 18305455. PMC PMC2683977. Research Blogging.
- ↑ Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP et al. (2005). "The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen.". Blood 106 (7): 2329-33. DOI:10.1182/blood-2005-03-1108. PMID 15947090. Research Blogging.
- ↑ Kamali F, Wynne H (2010). "Pharmacogenetics of warfarin.". Annu Rev Med 61: 63-75. PMID 19686083.
- ↑ FDA Approves Updated Warfarin (Coumadin) Prescribing Information. Retrieved on 2007-08-20.
- ↑ Kangelaris KN et al. (2009). "Genetic testing before anticoagulation? A systematic review of pharmacogenetic dosing of warfarin". J Gen Intern Med 24: 656–64. PMID 19306050.
- ↑ 16.0 16.1 Wadelius M et al (2005). "Common VKORC1 and GGCX polymorphisms associated with warfarin dose". Pharmacogenomics J 5: 262-70. PMID 15883587.
- ↑ Rost S et al (2004). "Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2". Nature 427: 537–41. PMID 14765194.
- ↑ 18.0 18.1 Rieder MJ et al (2005). "Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose". N Engl J Med 352: 2285-93. PMID 15930419.
- ↑ 19.0 19.1 Sanderson S, Emery J, Higgins J (2005). "CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis". Genet Med 7: 97-104. PMID 15714076.
- ↑ Dentali F et al. (2011). "Brand name versus generic warfarin: a systematic review of the literature.". Pharmacotherapy 31 (4): 386-93. PMID 21449627.
- ↑ Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ et al. (2012). "Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines.". Chest 141 (2 Suppl): e152S-84S. DOI:10.1378/chest.11-2295. PMID 22315259. PMC PMC3278055. Research Blogging.
- ↑ 22.0 22.1 Ansell J et al. (2008). "Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)". Chest 133 Suppl: 160S-98S. PMID 18574265.
- ↑ Blondon M, Righini M, Aujesky D, Le Gal G, Perrier A (2012). "Utility of preemptive anticoagulation in patients with suspected pulmonary embolism: a decision analysis.". Chest. DOI:10.1378/chest.11-2694. PMID 22383664. Research Blogging.
- ↑ 24.0 24.1 24.2 24.3 Kovacs MJ, Rodger M, Anderson DR, et al (2003). "Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial". Ann Intern Med 138: 714-9. PMID 12729425. [e]
- ↑ 25.0 25.1 25.2 Crowther MA et al. (1999). "A randomized trial comparing 5-mg and 10-mg warfarin loading doses". Arch Intern Med 159: 46–8. PMID 9892329.
- ↑ 26.0 26.1 26.2 26.3 Quiroz R et al. (2006). "Comparison of a single end point to determine optimal initial warfarin dosing (5 mg versus 10 mg) for venous thromboembolism". Am J Cardiol 98: 535–7. PMID 16893712.
- ↑ 27.0 27.1 27.2 27.3 Anderson JL et al. (2007). "Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation". Circulation 116: 2563–70. PMID 17989110.
- ↑ 28.0 28.1 28.2 28.3 Farahmand S, Saeedi M, Seyed Javadi HH, Khashayar P (2011). "High doses of warfarin are more beneficial than its low doses in patients with deep vein thrombosis.". Am J Emerg Med 29 (9): 1222-6. DOI:10.1016/j.ajem.2010.07.008. PMID 21035980. Research Blogging.
- ↑ 29.0 29.1 29.2 Caraco Y et al. (2008). "CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study". Clin Pharmacol Ther 83: 460–70. PMID 17851566.
- ↑ 30.0 30.1 30.2 Ansell J et al. (2004). "The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy". Chest 126 (3 Suppl): 204S–33S. PMID 15383473.
- ↑ Tait RC, Sefcick A (1998). "A warfarin induction regimen for out-patient anticoagulation in patients with atrial fibrillation". Br. J. Haematol. 101 (3): 450-4. DOI:10.1046/j.1365-2141.1998.00716.x. PMID 9633885. Research Blogging.
- ↑ Fennerty A et al. (1984). "Flexible induction dose regimen for warfarin and prediction of maintenance dose". Br Med J (Clin Res Ed) 288: 1268–70. PMID 6424820. [PubMed Central]
- ↑ Fennerty A et al. (1988). "Anticoagulants in venous thromboembolism". BMJ 297 (6659): 1285-8. PMID 3144365.
- ↑ Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF et al. (2013). "A Pharmacogenetic versus a Clinical Algorithm for Warfarin Dosing.". N Engl J Med. DOI:10.1056/NEJMoa1310669. PMID 24251361. Research Blogging.
- ↑ Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T et al. (2013). "A Randomized Trial of Genotype-Guided Dosing of Warfarin.". N Engl J Med. DOI:10.1056/NEJMoa1311386. PMID 24251363. Research Blogging.
- ↑ Klein TE et al. (2009). "Estimation of the warfarin dose with clinical and pharmacogenetic data". N. Engl. J. Med. 360: 753–64. PMID 19228618.
- ↑ Millican E et al. (2007). "Genetic-based dosing in orthopaedic patients beginning warfarin therapy" 110 (5): 1511-5. PMID 17387222. Online tool based on the study.
- ↑ Eckman MH et al. (2009). "Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation.". Ann Intern Med 150: 73-83. PMID 19153410.
- ↑ Eckman MH, et al. (2009). "Should we test for CYP2C9 before initiating anticoagulant therapy in patients with atrial fibrillation?". J Gen Intern Med 24: 543–9. PMID 19255811.
- ↑ Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ (2005). "Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population.". Heart 91 (4): 472-7. DOI:10.1136/hrt.2004.042465. PMID 15772203. PMC PMC1768813. Research Blogging.
- ↑ Rose AJ, Ozonoff A, Berlowitz DR, Henault LE, Hylek EM (January 2009). "Warfarin dose management affects INR control". J. Thromb. Haemost. 7 (1): 94–101. DOI:10.1111/j.1538-7836.2008.03199.x. PMID 18983486. Research Blogging.
- ↑ 42.0 42.1 van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ (2006). "Effect of study setting on anticoagulation control: a systematic review and metaregression.". Chest 129 (5): 1155-66. DOI:10.1378/chest.129.5.1155. PMID 16685005. Research Blogging.
- ↑ Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al. (2009). "Dabigatran versus warfarin in patients with atrial fibrillation.". N Engl J Med 361 (12): 1139-51. DOI:10.1056/NEJMoa0905561. PMID 19717844. Research Blogging. Review in: Ann Intern Med. 2010 Jan 19;152(2):JC1-2
- ↑ Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S et al. (2009). "Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran.". Am Heart J 157 (5): 805-10, 810.e1-2. DOI:10.1016/j.ahj.2009.02.005. PMID 19376304. Research Blogging.
- ↑ Van Spall HG, Wallentin L, Yusuf S, Eikelboom JW, Nieuwlaat R, Yang S et al. (2012). "Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries: an analysis of patients receiving warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial.". Circulation 126 (19): 2309-16. DOI:10.1161/CIRCULATIONAHA.112.101808. PMID 23027801. Research Blogging.
- ↑ Anderson JL, Horne BD, Stevens SM, Woller SC, Samuelson KM, Mansfield JW et al. (2012). "A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II).". Circulation 125 (16): 1997-2005. DOI:10.1161/CIRCULATIONAHA.111.070920. PMID 22431865. Research Blogging.
- ↑ Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M et al. (2011). "Apixaban versus warfarin in patients with atrial fibrillation.". N Engl J Med 365 (11): 981-92. DOI:10.1056/NEJMoa1107039. PMID 21870978. Research Blogging. Review in: Ann Intern Med. 2012 Jan 17;156(2):JC1-2, JC1-3
- ↑ Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD et al. (2010). "Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale.". Am Heart J 159 (3): 331-9. DOI:10.1016/j.ahj.2009.07.035. PMID 20211292. Research Blogging.
- ↑ Torn M et al. (July 2009). "Optimal level of oral anticoagulant therapy for the prevention of arterial thrombosis in patients with mechanical heart valve prostheses, atrial fibrillation, or myocardial infarction: a prospective study of 4202 patients". Arch Intern Med 169: 1203–9. PMID 19597069.
- ↑ Heneghan, Carl; Alison Ward, Rafael Perera (2011-12-01). "Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data". The Lancet. ISSN 0140-6736.
- ↑ Schulman S, Parpia S, Stewart C, Rudd-Scott L, Julian JA, Levine M (2011). "Warfarin dose assessment every 4 weeks versus every 12 weeks in patients with stable international normalized ratios: a randomized trial.". Ann Intern Med 155 (10): 653-9. DOI:10.1059/0003-4819-155-10-201111150-00003. PMID 22084331. Research Blogging.
- ↑ Kearon C, Hirsh J (1997). "Management of anticoagulation before and after elective surgery". N Engl J Med 336: 1506–11. PMID 9154771.
- ↑ Pengo V, Cucchini U, Denas G, et al. (2009). "Standardized low-molecular-weight heparin bridging regimen in outpatients on oral anticoagulants undergoing invasive procedure or surgery: an inception cohort management study". Circulation 119: 2920–7. PMID 19470892.
- ↑ Garcia DA et al. (2008). "Risk of thromboembolism with short-term interruption of warfarin therapy". Arch Intern Med 168: 63–9. PMID 18195197.
- ↑ Douketis JD et al. (2008). "The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)". Chest 133 (6 Suppl): 299S-339S. PMID 18574269.
- ↑ Douketis JD, Johnson JA, Turpie AG (June 2004). "Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen". Arch Intern Med 164: 1319–26. PMID 15226166.
- ↑ Whitlock RP et al. (2007). "Warfarin cessation before cardiopulmonary bypass: lessons learned from a randomized controlled trial of oral vitamin K". Ann Thorac Surg 84 (1): 103–8. PMID 17588394.
- ↑ Witt DM et al. (2009). "Incidence and predictors of bleeding or thrombosis after polypectomy in patients receiving and not receiving anticoagulation therapy.". J Thromb Haemost 7: 1982-9. PMID 19719825.
- ↑ Bell DF et al. (1988). "Elevated partial thromboplastin time as an indicator of hemorrhagic risk in postoperative patients on warfarin prophylaxis". J Arthroplasty 3: 181–4. PMID 3397749.
- ↑ Hauser VM, Rozek SL (1986). "Effect of warfarin on the activated partial thromboplastin time". Drug Intell Clin Pharm 20: 964–7. PMID 3816546.
- ↑ Mega, Jessica (2011 [last update]). A New Era for Anticoagulation in Atrial Fibrillation — NEJM. nejm.org. Retrieved on August 29, 2011.
- ↑ Samsa GP, Matchar DB, Goldstein LB, Bonito AJ, Lux LJ, Witter DM et al. (2000). "Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities.". Arch Intern Med 160 (7): 967-73. PMID 10761962. [e]
- ↑ 63.0 63.1 McBride D, Brüggenjürgen B, Roll S, Willich SN (2007). "Anticoagulation treatment for the reduction of stroke in atrial fibrillation: a cohort study to examine the gap between guidelines and routine medical practice.". J Thromb Thrombolysis 24 (1): 65-72. DOI:10.1007/s11239-006-0002-8. PMID 17260164. Research Blogging.
- ↑ 64.0 64.1 Pengo V, Pegoraro C, Cucchini U, Iliceto S (2006). "Worldwide management of oral anticoagulant therapy: the ISAM study.". J Thromb Thrombolysis 21 (1): 73-7. DOI:10.1007/s11239-006-5580-y. PMID 16475046. Research Blogging.
- ↑ Wieloch M, Själander A, Frykman V, Rosenqvist M, Eriksson N, Svensson PJ (2011). "Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA.". Eur Heart J 32 (18): 2282-9. DOI:10.1093/eurheartj/ehr134. PMID 21616951. Research Blogging.
- ↑ Juurlink DN (2007). "Drug interactions with warfarin: what clinicians need to know". CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 177: 369–71. PMID 17698826.
- ↑ Holbrook AM et al. (2005). "Systematic overview of warfarin and its drug and food interactions". Arch. Intern Med. 165: 1095–106. PMID 15911722.
- ↑ Lopes RD, Crowley MJ, Shah BR, et al . Stroke Prevention in Atrial Fibrillation. Comparative Effectiveness Review No. 123. AHRQ Publication No. 13-EHC113-EF. Rockville, MD: Agency for Healthcare Research and Quality; August 2013. http://www.effectivehealthcare.ahrq.gov/ reports/final.cfm.
- ↑ Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N et al. (2011). "A New Risk Scheme to Predict Warfarin-Associated Hemorrhage The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study.". J Am Coll Cardiol 58 (4): 395-401. DOI:10.1016/j.jacc.2011.03.031. PMID 21757117. Research Blogging.
- ↑ Hylek EM et al. (2007). "Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation". Circulation 115: 2689-96. PMID 17515465.
- ↑ Claassen DO et al. (2008). "Restarting anticoagulation therapy after warfarin-associated intracerebral hemorrhage". Arch Neurol 65: 1313–8. PMID 18852344.
- ↑ 72.0 72.1 Prandoni P, Lensing AW, Piccioli A, et al (2002). "Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis". Blood 100 (10): 3484–8. DOI:10.1182/blood-2002-01-0108. PMID 12393647. Research Blogging.
- ↑ Sconce E et al. (2007). "Vitamin K supplementation can improve stability of anticoagulation for patients with unexplained variability in response to warfarin". Blood 109: 2419–23. PMID 17110451.
- ↑ 1Crowther, MA; et al. (2009-03-03). "Oral Vitamin K Versus Placebo to Correct Excessive Anticoagulation in Patients Receiving Warfarin: A Randomized Trial". Ann Intern Med 150: 293-300.
- ↑ Crowther MA et al. (2002). "Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial". Ann Intern Med 137: 251-4. PMID 12186515.
- ↑ Dezee KJ et al. (2006). "Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis". Arch Intern Med 166: 391–7. PMID 16505257.
- ↑ Hughes GJ, Patel PN, Saxena N.. Effect of Acetaminophen on International Normalized Ratio in Patients Receiving Warfarin Therapy. Pharmacotherapy 2011 June;31(6):591–597.
- ↑ Zhang Q et al. (2011). "Interaction between acetaminophen and warfarin in adults receiving long-term oral anticoagulants: a randomized controlled trial.". Eur J Clin Pharmacol 67: 309-14. PMID 21191575.
- ↑ Johnson SG et al. (2008). "Outcomes associated with combined antiplatelet and anticoagulant therapy". Chest 133 (4): 948–54. PMID 18198244.
- ↑ Schelleman H et al. (2010). "Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk.". Am J Med 123: 151-7. PMID 20103024.
- ↑ Fischer, HD; et al. (2010-04-12). "Hemorrhage during warfarin therapy associated with cotrimoxazole and other urinary tract anti-infective agents: a population-based study". Arch Intern Med 170: 617-621. DOI:10.1001/archinternmed.2010.37. Research Blogging.