Atazanavir: Difference between revisions
imported>David E. Volk mNo edit summary |
mNo edit summary |
||
| (8 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Atazanavir structure.jpg| | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Atazanavir structure.jpg|center|thumb|275px]] | |||
|width=275px | |||
|molname=atazanavir | |||
|synonyms= AZT | |||
|molformula= C<sub>38</sub>H<sub>52</sub>N<sub>6</sub>O<sub>7</sub> | |||
|molmass= 704.8555 | |||
|uses=HIV | |||
|properties=protease inhibitor | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber= 198904-31-3 | |||
}} | |||
'''Atazanavir''', widely known as '''ATZ''', and also called '''ATV''' and formerly called '''BMS-232632''', is an antiretroviral [[protease inhibitor]] (PI) used to treat [[HIV]]/[[AIDS]]. Unlike most protease inhibitors, atazanavir can be given once a day and hence has fewer effects on patient lipid profiles. Like most protease inhibitors, it is used in combination with other HIV medications. | '''Atazanavir''', widely known as '''ATZ''', and also called '''ATV''' and formerly called '''BMS-232632''', is an antiretroviral [[protease inhibitor]] (PI) used to treat [[HIV]]/[[AIDS]]. Unlike most protease inhibitors, atazanavir can be given once a day and hence has fewer effects on patient lipid profiles. Like most protease inhibitors, it is used in combination with other HIV medications. | ||
It is sold under the brand names Latazanavir®, Reyataz® and Zrivada®. | It is sold under the brand names Latazanavir®, Reyataz® and Zrivada®. | ||
== Mechanism of action == | |||
It inhibits the proteolytic cleavage of the viral polyprotein precursors (Gag and Gag-Pol) so that the individual active proteins of HIV cannot be made, thus stopping the formation of mature, infectious viral particles. This and other protease inhibitors are almost always used in combination with at least two other anti-HIV drugs. | |||
== Chemistry == | |||
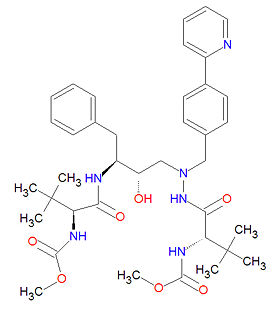
Its IUPAC chemical name is methyl N-[(2S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)- | Its IUPAC chemical name is methyl N-[(2S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)- | ||
3,3-dimethylbutanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenylbutan-2-yl]amino]-3,3-dimethyl-1-oxobutan-2-yl]carbamate, and its chemical formula is C<sub>38</sub>H<sub>52</sub>N<sub>6</sub>O<sub>7</sub> (MW 704.8555 g/mol). | 3,3-dimethylbutanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenylbutan-2-yl]amino]-3,3-dimethyl-1-oxobutan-2-yl]carbamate, and its chemical formula is C<sub>38</sub>H<sub>52</sub>N<sub>6</sub>O<sub>7</sub> (MW 704.8555 g/mol). | ||
| Line 11: | Line 30: | ||
== Drug interactions == | == Drug interactions == | ||
The absorption of indinavir is decreased by [[St. John's Wort]], antacids and other gastric pH modifiers, such as [[ | The absorption of indinavir is decreased by [[St. John's Wort]], antacids and other gastric pH modifiers, such as [[aluminium]], [[bismuth]], [[calcium]], [[cimetidine]], [[dihydroxyaluminium]], [[famotidine]], [[magnesium]], [[magnesium oxide]], [[magnesium sulfide]], [[nizatidine]], [[ranitidine]] and [[sodium bicarbonate]], by [[efavirenz]], [[omeprazole]] and related compounds ([[esomeprazole]], [[lansoprazole]], [[pantoprazole]] and [[rabeprazole]]) and by [[rifampin]] and its derivitive [[rifabutin]]. It is also decreased when taking [[nevirapine]] or [[tenofovir]]. | ||
Atazanavir increases the anticoagulant effect of [[anisindione]], [[acenocoumarol]], [[dicumarol]], and [[warfarin]] and also increases the effect of [[benzodiazepine]] when taken with [[midazolam]] or [[triazolam]]. An increased risk of cardiotoxicity and [[arrhythmia]]s occurs when taken with [[amiodarone]], [[cisapride]], [[dihydroquinidine barbiturate]], [[lidocaine]], [[quinidine]], and [[quinidine barbiturate]]. | Atazanavir increases the anticoagulant effect of [[anisindione]], [[acenocoumarol]], [[dicumarol]], and [[warfarin]] and also increases the effect of [[benzodiazepine]] when taken with [[midazolam]] or [[triazolam]]. An increased risk of cardiotoxicity and [[arrhythmia]]s occurs when taken with [[amiodarone]], [[cisapride]], [[dihydroquinidine barbiturate]], [[lidocaine]], [[quinidine]], and [[quinidine barbiturate]]. | ||
| Line 18: | Line 37: | ||
== External Links == | == External Links == | ||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 14 July 2024
|
| |||||||
| atazanavir | |||||||
| |||||||
| Uses: | HIV | ||||||
| Properties: | protease inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Atazanavir, widely known as ATZ, and also called ATV and formerly called BMS-232632, is an antiretroviral protease inhibitor (PI) used to treat HIV/AIDS. Unlike most protease inhibitors, atazanavir can be given once a day and hence has fewer effects on patient lipid profiles. Like most protease inhibitors, it is used in combination with other HIV medications.
It is sold under the brand names Latazanavir®, Reyataz® and Zrivada®.
Mechanism of action
It inhibits the proteolytic cleavage of the viral polyprotein precursors (Gag and Gag-Pol) so that the individual active proteins of HIV cannot be made, thus stopping the formation of mature, infectious viral particles. This and other protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
Chemistry
Its IUPAC chemical name is methyl N-[(2S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)- 3,3-dimethylbutanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenylbutan-2-yl]amino]-3,3-dimethyl-1-oxobutan-2-yl]carbamate, and its chemical formula is C38H52N6O7 (MW 704.8555 g/mol).
Drug interactions
The absorption of indinavir is decreased by St. John's Wort, antacids and other gastric pH modifiers, such as aluminium, bismuth, calcium, cimetidine, dihydroxyaluminium, famotidine, magnesium, magnesium oxide, magnesium sulfide, nizatidine, ranitidine and sodium bicarbonate, by efavirenz, omeprazole and related compounds (esomeprazole, lansoprazole, pantoprazole and rabeprazole) and by rifampin and its derivitive rifabutin. It is also decreased when taking nevirapine or tenofovir.
Atazanavir increases the anticoagulant effect of anisindione, acenocoumarol, dicumarol, and warfarin and also increases the effect of benzodiazepine when taken with midazolam or triazolam. An increased risk of cardiotoxicity and arrhythmias occurs when taken with amiodarone, cisapride, dihydroquinidine barbiturate, lidocaine, quinidine, and quinidine barbiturate.
The effects and toxicity of some statins, such as atorvastatin, lovastatin and simvastatin, is increased when taking atazanavir. The effects of cyclosporine, pimozide, sildenafil and tacrolimus are increased when taken with atazanavir. The effect and toxicity of ergot derivatives, such as ergotamine, dihydroergotamine and methylergonovine, and the drugs erlotinib and ranolazine are also increased. The effects and toxicity of tricyclic compounds are increased when atazanavir is taken with amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline, or trimipramine. The effects or levels of the following are also increased: bepridil, clarithromycin, diltiazem, irinotecan, sirolimus and sunitinib. Increased risk of hyperbilirubinemia exists when indinavir and atazanavir are taken together.
External Links
The most up-to-date information about Atazanavir and other drugs can be found at the following sites.
- Atazanavir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Atazanavir - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Atazanavir - Detailed information from DrugBank.