Ketoconazole: Difference between revisions
imported>David E. Volk (→Conditions with increased risks: ergotamines) |
mNo edit summary |
||
| (40 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
'''Ketoconazole''' is one of the [[azole]]-based [[antifungal drug]]s used to treat fungal infections. Its use has greatly diminished because of the introduction of better treatment alternatives, including the | {{Chem infobox | ||
|align=right | |||
|image=[[Image:Ketoconazole structure.jpg|center|thumb|350px]] | |||
|width=350px | |||
|molname=ketoconazole | |||
|synonyms= many, see below | |||
|molformula= C<sub>26</sub>H<sub>28</sub>Cl<sub>2</sub>N<sub>4</sub>O<sub>4</sub> | |||
|molmass= 531.43 | |||
|uses=antifungal drug | |||
|properties=azole compound | |||
|hazards=see side effects & drug interactions | |||
|iupac= see chemistry section | |||
|casnumber= 79156-75-5 | |||
}} | |||
'''Ketoconazole''' is one of the [[azole]]-based [[antifungal drug]]s used to treat [[fungus|fungal]] infections. Its use has greatly diminished because of the introduction of better treatment alternatives, including the [[triazole]]s [[fluconazole]] and [[itraconazole]]. It is based on [[imidazole]] as are [[clotrimazole]], fluconazole, itraconazole and [[miconazole]]. It is a second-line of defense systemic drug used to treat [[candidiasis]], [[candiduria]], [[oral thrush]], [[mucocutaneious candidiasis]], [[blastomycosis]], [[coccidioidomycosis]], [[histoplasmosis]], [[chromomycosis]] and [[paracocccidioidomycosis]]. Typical fungal infections like [[athlete's foot]], [[jock itch]] and [[ringworm]] can be treated with ketoconazole. Side effects associated with erectile dysfunction and steroid synthesis broaden the usages of this drug. Fungal meningitis should not be treated with ketoconazole because it does not localize into the cerebral spinal fluid well. | |||
== Mechanism of action == | == Mechanism of action == | ||
Azole-based antifungal agents, such as ketoconazole, work by inhibiting the enzyme cytochrome P450 14-<math>\alpha</math>-demethylase (P45014DM), which is part of the sterol biosynthesis pathway that converts [[lanosterol]] to [[ergosterol]]<ref name=Lyman>{{cite journal | journal = Drugs | title = Systemically administered antifungal agents. A review of the their clinical pharmacology and therapeutic applications | authors = Lyman, C. A. and Walsh, T. J. | volume = 44 | pages = 9-35 | year = 1992 }}</ref>. Because ketoconazole has less affinity towards fungal cell membranes than the newer triazole antifungal agents like fluconazole and itraconazole, it is more likely to bind with mammalian cell membranes and induce toxicity<ref name=Como>{{cite journal | journal = N. Engl. J. Med. | title = Oral azole drugs as systemic antifungal therapy | authors = Como, J. A. and Dismuke, W. E. | volume = 330 | pages = 263-272 | year = 1994 }}</ref>. | Azole-based antifungal agents, such as ketoconazole, work by inhibiting the enzyme cytochrome P450 14-<math>\alpha</math>-demethylase (P45014DM), which is part of the sterol biosynthesis pathway that converts [[lanosterol]] to [[ergosterol]]<ref name=Lyman>{{cite journal | journal = Drugs | title = Systemically administered antifungal agents. A review of the their clinical pharmacology and therapeutic applications | authors = Lyman, C. A. and Walsh, T. J. | volume = 44 | pages = 9-35 | year = 1992 }}</ref>. Because ketoconazole has less affinity towards fungal cell membranes than the newer triazole antifungal agents like fluconazole and itraconazole, it is more likely to bind with mammalian cell membranes and induce toxicity<ref name=Como>{{cite journal | journal = N. Engl. J. Med. | title = Oral azole drugs as systemic antifungal therapy | authors = Como, J. A. and Dismuke, W. E. | volume = 330 | pages = 263-272 | year = 1994 }}</ref>. | ||
== Side effects == | == Side effects == | ||
Because ketoconazole disrupts part of the sterol biosynthesis pathway, it may decrease levels of the [[steroid]]s [[testosterone]] and [[cortisone]] causing [[gynecomastia]] and [[oligospermia]] in males and irregular [[menstruation]] in women. It may also cause [[anorexia]], nausea and vomiting, increased levels of [[transaminase]] and [[hepatic toxicity|liver toxicity]]. | |||
== Chemistry == | == Chemistry == | ||
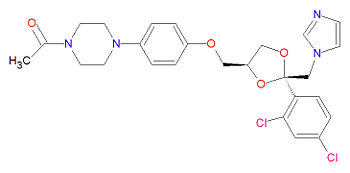
The IUPAC chemical name for ketoconazole is 1-[4-[4-[[2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone. It has chemical formula C<sub>26</sub>H<sub>28</sub>C<sub>l2</sub>N<sub>4</sub>O<sub>4</sub>, a molecular mass of 531.4309 g/mol and is registered under CAS Number 79156-75-5. | The IUPAC chemical name for ketoconazole is 1-[4-[4-[[2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone. It has chemical formula C<sub>26</sub>H<sub>28</sub>C<sub>l2</sub>N<sub>4</sub>O<sub>4</sub>, a molecular mass of 531.4309 g/mol and is registered under CAS Number 79156-75-5. The activity of the drug is based on the presence of [[imidazole]], an [[azole]]. Ketoconazole also contains a [[piperazine]] ring system and is an [[antiandrogen]] compound. | ||
== Drug interactions == | == Drug interactions == | ||
Ketoconazole can have many drug interactions, including interacting with medicines or natural compounds related to [[diabetes]], [[cardiac disease]], [[psychological]] diseases, [[antiviral drug]]s, [[erectile dysfunction]], [[vascular disease]], [[steroid]]-related diseases, [[migraine headache]]s, [[sleep disorder]]s and others. See the following sections for specific interactions and conditions with increased risk factors. | |||
=== | === Drugs enhanced by ketoconazole === | ||
Ketoconazole increases the effectiveness, and therefore toxicity of many medications. It increases the effectiveness of some anticoagualant medications, including [[acenocoumarol]], [[anisindione]], [[dicumarol]] and [[warfarin]] and the effectiveness and toxicity of some [[steroid]]s and steroid agonists, including the [[corticosteroid]]s [[methylprednisolone]], [[prednisolone]] and [[prednisone]], the [[glucocorticoid]]s [[budesonide]] and [[ciclesonide]] and the [[aldosterone]] agonist [[eplerenone]]. It also increase the effects of some [[antiviral drug]]s, including [[indinavir]], [[ritonavir]] and [[saquinavir]]. Many [[benzodiazepine]]-based drugs, including [[alazepam]], [[alprazolam]], [[chlordiazepoxide]], [[clonazepam]], [[clorazepate]], [[diazepam]], [[estazolam]], [[flurazepam]], [[midazolam]], [[quazepam]] and [[triazolam]] are more effective when taken with ketoconazole. The immunosuppressants [[cyclosporine]], [[everolimus]], [[sirolimus]] and [[tacrolimus]], and the tricyclic drugs [[amitriptyline]], [[imipramine]] and [[nortriptyline]], are enhanced when taken with ketoconazole. Because ketoconazole is a potent [[CYP3A4]] inhibitor, it increases the effects and toxicity of [[almotriptan]], [[aprepitant]], [[dofetilide]], [[eletriptan]], [[erlotinib]], [[gefitinib]], [[trazodone]], and also slows the metabolism of [[darifenacin]] and [[solifenacin]]. Drugs used to treat erectile dysfunction, like [[sildenafil]] (Viagra®, Revatio®), [[tadalafil]] (Cialis®) and [[vardenafil]] (Levitra®), and drugs used to treat [[diabetes]], such as [[pioglitazone]], [[rosiglitazone]] and [[ | Ketoconazole increases the effectiveness, and therefore toxicity of many medications. It increases the effectiveness of some anticoagualant medications, including [[acenocoumarol]], [[anisindione]], [[dicumarol]] and [[warfarin]] and the effectiveness and toxicity of some [[steroid]]s and steroid agonists, including the [[corticosteroid]]s [[methylprednisolone]], [[prednisolone]] and [[prednisone]], the [[glucocorticoid]]s [[budesonide]] and [[ciclesonide]] and the [[aldosterone]] agonist [[eplerenone]]. It also increase the effects of some [[antiviral drug]]s, including [[indinavir]], [[ritonavir]] and [[saquinavir]]. Many [[benzodiazepine]]-based drugs, including [[alazepam]], [[alprazolam]], [[chlordiazepoxide]], [[clonazepam]], [[clorazepate]], [[diazepam]], [[estazolam]], [[flurazepam]], [[midazolam]], [[quazepam]] and [[triazolam]] are more effective when taken with ketoconazole. The immunosuppressants [[cyclosporine]], [[everolimus]], [[sirolimus]] and [[tacrolimus]], and the tricyclic drugs [[amitriptyline]], [[imipramine]] and [[nortriptyline]], are enhanced when taken with ketoconazole. Because ketoconazole is a potent [[CYP3A4]] inhibitor, it increases the effects and toxicity of [[almotriptan]], [[aprepitant]], [[dofetilide]], [[eletriptan]], [[erlotinib]], [[gefitinib]], [[trazodone]], and also slows the metabolism of [[darifenacin]] and [[solifenacin]]. Drugs used to treat erectile dysfunction, like [[sildenafil]] (Viagra®, Revatio®), [[tadalafil]] (Cialis®) and [[vardenafil]] (Levitra®), and drugs used to treat [[diabetes]], such as [[pioglitazone]], [[rosiglitazone]] and [[tolbutamide]] have increased effects when taken with ketoconazole. The [[psycholeptic]] drugs [[aripiprazole]], [[buspirone]], [[galantamine]], [[haloperidol]], [[quetiapine]] and [[ziprasidone]] are likewise more effective. Ketoconazole also increases the levels of [[antineoplastic]] agents (antitumor drugs) including [[imatinib]], [[vinblastine]], [[vincristine]], [[docetaxel]], [[irinotecan]] the cardiac or vascular drugs [[bosentan]], [[cilostazol]], [[quinidine]] and [[ranolazine]], and [[analgesic]]s including [[fentanyl]] and [[alfentanil]]. The following may also be increased: [[carbamazepine]], [[cinacalcet]], [[ramelteon]], [[quinidine]], [[tolterodine]], [[valdecoxib]], [[sibutramine]], [[sunitinib]], and [[alfuzosin]]. | ||
== Drugs | === Drugs suppressed by ketoconazole === | ||
Some oral [[contraceptive]]s, including [[ethinyl estradiol]] and [[mestranol]] may be less effective when taken in combination with ketoconazole. | Some oral [[contraceptive]]s, including [[ethinyl estradiol]] and [[mestranol]] may be less effective when taken in combination with ketoconazole. | ||
=== Conditions with increased risks === | === Conditions with increased risks === | ||
The risk of [[myopathy]] and [[rhabdomyolysis]] is increased when ketoconazole is taken with some cholesterol-lowering [[statin]] drugs such as [[atorvastatin]], [[cerivastatin]], [[lovastatin]] and [[simvastatin]]. The risk of [[arrhythmia]]s and [[cardiotoxicity]] increase when this drug is taken with [[astemizole]], [[cisapride]], [[terfenadine]], [[pimozide]]. An increased risk of ergotism and severe ischemia may result when ketoconazole is taken with [[ergotamine]] and its derivate [[dihydroergotamine]]. | The risk of [[myopathy]] and [[rhabdomyolysis]] is increased when ketoconazole is taken with some cholesterol-lowering [[statin]] drugs such as [[atorvastatin]], [[cerivastatin]], [[lovastatin]] and [[simvastatin]]. The risk of [[arrhythmia]]s and [[cardiotoxicity]] increase when this drug is taken with [[astemizole]], [[cisapride]], [[terfenadine]], [[pimozide]]. An increased risk of [[ergotism]] and severe [[ischemia]] may result when ketoconazole is taken with [[ergotamine]] and its derivate [[dihydroergotamine]]. | ||
=== Drugs | === Drugs that impede ketoconazole === | ||
Antacids, including basic forms of [[aluminium]], [[bismuth]], [[calcium]] and [[magnesium]] , as well as the [[proton pump inhibitor]]s [[esomeprazole]], [[lansoprazole]], [[omeprazole]], [[pantoprazole]] and [[rabeprazole]] decrease the absorption of imidazole and hence make ketoconazole less effective. Likewise, the anti-H2 drugs [[cimetidine]], [[famotidine]], [[nizatidine]] and [[ranitidine]] | Antacids, including basic forms of [[aluminium]], [[bismuth]], [[calcium]] and [[magnesium]], as well as the [[proton pump inhibitor]]s [[esomeprazole]], [[lansoprazole]], [[omeprazole]], [[pantoprazole]] and [[rabeprazole]] decrease the absorption of imidazole and hence make ketoconazole less effective. Likewise, the anti-H2 drugs [[cimetidine]], [[famotidine]], [[nizatidine]] and [[ranitidine]] suppress ketoconazole absorption. Additional medications with this side effect include [[isoniazid]], [[nevirapine]], [[rifampin]] and [[sucralfate]]. | ||
== Synonyms == | |||
* (+-)-cis-1-acetyl-4-(p-((2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazine | |||
{{col-begin}} | |||
{{col-break|width=50%}} | |||
*79156-75-5 | *79156-75-5 | ||
* CPD-4503 | * CPD-4503 | ||
* fungarest | * fungarest | ||
* fungoral | * fungoral | ||
* ketoconazol | * ketoconazol | ||
* ketoconazole | * ketoconazole | ||
* ketoderm | * ketoderm | ||
* ketoisdin | {{col-break|width=50%}} | ||
* ketoisdin | |||
* KW 1414 | * KW 1414 | ||
* NCI60_002728 | * NCI60_002728 | ||
| Line 45: | Line 61: | ||
* orifungal M | * orifungal M | ||
* panfungol | * panfungol | ||
{{col-end}} | |||
== Brand names == | == Brand names == | ||
{{col-begin}} | |||
{{col-break|width=50%}} | |||
* Extina® | * Extina® | ||
* Fungarest® | * Fungarest® | ||
| Line 57: | Line 76: | ||
* Ketoderm® | * Ketoderm® | ||
* Ketoisdin® | * Ketoisdin® | ||
{{col-break|width=50%}} | |||
* Ketozole® | * Ketozole® | ||
* Nizoral® | * Nizoral® | ||
| Line 66: | Line 86: | ||
* Orifungal M® | * Orifungal M® | ||
* Panfungol® | * Panfungol® | ||
{{col-end}} | |||
== References == | == References == | ||
<references/> | <references/> | ||
== Primary Sources == | |||
* Doctorfungus [http://www.doctorfungus.org/thedrugs/Ketoconazole.htm] | |||
* {{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 8 September 2024
|
| |||||||
| ketoconazole | |||||||
| |||||||
| Uses: | antifungal drug | ||||||
| Properties: | azole compound | ||||||
| Hazards: | see side effects & drug interactions | ||||||
| |||||||
Ketoconazole is one of the azole-based antifungal drugs used to treat fungal infections. Its use has greatly diminished because of the introduction of better treatment alternatives, including the triazoles fluconazole and itraconazole. It is based on imidazole as are clotrimazole, fluconazole, itraconazole and miconazole. It is a second-line of defense systemic drug used to treat candidiasis, candiduria, oral thrush, mucocutaneious candidiasis, blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis and paracocccidioidomycosis. Typical fungal infections like athlete's foot, jock itch and ringworm can be treated with ketoconazole. Side effects associated with erectile dysfunction and steroid synthesis broaden the usages of this drug. Fungal meningitis should not be treated with ketoconazole because it does not localize into the cerebral spinal fluid well.
Mechanism of action
Azole-based antifungal agents, such as ketoconazole, work by inhibiting the enzyme cytochrome P450 14--demethylase (P45014DM), which is part of the sterol biosynthesis pathway that converts lanosterol to ergosterol[1]. Because ketoconazole has less affinity towards fungal cell membranes than the newer triazole antifungal agents like fluconazole and itraconazole, it is more likely to bind with mammalian cell membranes and induce toxicity[2].
Side effects
Because ketoconazole disrupts part of the sterol biosynthesis pathway, it may decrease levels of the steroids testosterone and cortisone causing gynecomastia and oligospermia in males and irregular menstruation in women. It may also cause anorexia, nausea and vomiting, increased levels of transaminase and liver toxicity.
Chemistry
The IUPAC chemical name for ketoconazole is 1-[4-[4-[[2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone. It has chemical formula C26H28Cl2N4O4, a molecular mass of 531.4309 g/mol and is registered under CAS Number 79156-75-5. The activity of the drug is based on the presence of imidazole, an azole. Ketoconazole also contains a piperazine ring system and is an antiandrogen compound.
Drug interactions
Ketoconazole can have many drug interactions, including interacting with medicines or natural compounds related to diabetes, cardiac disease, psychological diseases, antiviral drugs, erectile dysfunction, vascular disease, steroid-related diseases, migraine headaches, sleep disorders and others. See the following sections for specific interactions and conditions with increased risk factors.
Drugs enhanced by ketoconazole
Ketoconazole increases the effectiveness, and therefore toxicity of many medications. It increases the effectiveness of some anticoagualant medications, including acenocoumarol, anisindione, dicumarol and warfarin and the effectiveness and toxicity of some steroids and steroid agonists, including the corticosteroids methylprednisolone, prednisolone and prednisone, the glucocorticoids budesonide and ciclesonide and the aldosterone agonist eplerenone. It also increase the effects of some antiviral drugs, including indinavir, ritonavir and saquinavir. Many benzodiazepine-based drugs, including alazepam, alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, midazolam, quazepam and triazolam are more effective when taken with ketoconazole. The immunosuppressants cyclosporine, everolimus, sirolimus and tacrolimus, and the tricyclic drugs amitriptyline, imipramine and nortriptyline, are enhanced when taken with ketoconazole. Because ketoconazole is a potent CYP3A4 inhibitor, it increases the effects and toxicity of almotriptan, aprepitant, dofetilide, eletriptan, erlotinib, gefitinib, trazodone, and also slows the metabolism of darifenacin and solifenacin. Drugs used to treat erectile dysfunction, like sildenafil (Viagra®, Revatio®), tadalafil (Cialis®) and vardenafil (Levitra®), and drugs used to treat diabetes, such as pioglitazone, rosiglitazone and tolbutamide have increased effects when taken with ketoconazole. The psycholeptic drugs aripiprazole, buspirone, galantamine, haloperidol, quetiapine and ziprasidone are likewise more effective. Ketoconazole also increases the levels of antineoplastic agents (antitumor drugs) including imatinib, vinblastine, vincristine, docetaxel, irinotecan the cardiac or vascular drugs bosentan, cilostazol, quinidine and ranolazine, and analgesics including fentanyl and alfentanil. The following may also be increased: carbamazepine, cinacalcet, ramelteon, quinidine, tolterodine, valdecoxib, sibutramine, sunitinib, and alfuzosin.
Drugs suppressed by ketoconazole
Some oral contraceptives, including ethinyl estradiol and mestranol may be less effective when taken in combination with ketoconazole.
Conditions with increased risks
The risk of myopathy and rhabdomyolysis is increased when ketoconazole is taken with some cholesterol-lowering statin drugs such as atorvastatin, cerivastatin, lovastatin and simvastatin. The risk of arrhythmias and cardiotoxicity increase when this drug is taken with astemizole, cisapride, terfenadine, pimozide. An increased risk of ergotism and severe ischemia may result when ketoconazole is taken with ergotamine and its derivate dihydroergotamine.
Drugs that impede ketoconazole
Antacids, including basic forms of aluminium, bismuth, calcium and magnesium, as well as the proton pump inhibitors esomeprazole, lansoprazole, omeprazole, pantoprazole and rabeprazole decrease the absorption of imidazole and hence make ketoconazole less effective. Likewise, the anti-H2 drugs cimetidine, famotidine, nizatidine and ranitidine suppress ketoconazole absorption. Additional medications with this side effect include isoniazid, nevirapine, rifampin and sucralfate.
Synonyms
- (+-)-cis-1-acetyl-4-(p-((2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazine
|
|
Brand names
|
|
References
Primary Sources
- Doctorfungus [1]
- The most up-to-date information about Ketoconazole and other drugs can be found at the following sites.
- Ketoconazole - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Ketoconazole - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Ketoconazole - Detailed information from DrugBank.