Michael Faraday: Difference between revisions
imported>Paul Wormer m (Minor tweaks in lead-in) |
mNo edit summary |
||
| (77 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Faraday.jpg|thumb|350px|Michael Faraday. Photograph by John Watkins, ca. 1861]] | |||
'''Michael Faraday''' (September 22, 1791 – August 25, 1867) was an English physicist and chemist who is one of the most influential scientists of all time.<ref>Simmons, John G. ''The Scientific 100: A Ranking of the Most Influential Scientists, Past and Present'' | [http://tinyurl.com/yku8v8w Complete chapter on Michael Faraday, pages 59-63.] Google Books preview.</ref> His most important contributions, and best known work, were on the closely connected phenomena of [[electricity]] and [[magnetism]], but he also made very significant contributions in chemistry. | |||
Faraday was principally an experimentalist; in fact, he has been described as the "best experimentalist in the history of science".<ref>[http://www.bath.ac.uk/news/2006/10/25/gulp-ford251006.html] Press Release, University of Bath, 25 October 2006</ref> He did not know any advanced mathematics, however.<ref>As a result he has been likened to [[Moses]], in that he brought the scientific fields he worked in to a place he himself could not enter: an age when advanced mathematics became the language of science. See Simmons, John G. ''The Scientific 100: A Ranking of the Most Influential Scientists, Past and Present'', pp. 59-60.</ref> Both his contributions to science, and his impact on the world, are nonetheless vast: his scientific discoveries underlie significant areas of modern physics and chemistry, and the technology which evolved from his work is even more widespread. His discoveries in [[electromagnetism]] laid the groundwork for the engineering work in the late 1800s by people such as [[Thomas Edison|Edison]], [[Werner von Siemens|Siemens]], [[Nikola Tesla|Tesla]] and [[George Westinghouse|Westinghouse]], <!-- Those 4 are listed in alphabetic order to avoid a debate over the relative magnitude of their contributions. --> which brought about the [[electrification]] of industrial societies, and his work in [[electrochemistry]] is now widely used in the field of [[chemical engineering]]. | |||
[[ | In [[physics]], he was one of the first to explore the ways in which electricity and magnetism are connected. In 1821, shortly after [[Hans Christian Oersted|Oersted]] first discovered that electricity and magnetism were associated, Faraday published his work on what he called ''electromagnetic rotation'' (the principle behind the [[electric motor]]). In 1831, Faraday discovered [[electromagnetic induction]], the principle behind the [[electric generator]] and [[electric transformer]]. His ideas about electrical and magnetic [[fields]], and the nature of fields in general, inspired later work in this area (such as [[Maxwell's equations]]), and fields of the type he envisaged are a key concept in today's physics. | ||
In chemistry, he created the first known compounds of [[carbon]] and [[chlorine]], helped to lay the foundations of [[metallurgy]] and [[metallography]], succeeded in liquifying a number of gasses for the first time, and discovered [[benzene]]. Perhaps his biggest contribution was in virtually founding electrochemistry, and introducing terminology such as [[electrolyte]], [[anode]], [[cathode]], [[electrode]], and [[ion]]. <ref>Simmons, John G. ''The Scientific 100: A Ranking of the Most Influential Scientists, Past and Present'', pg. 62. </ref> | |||
{{TOC|right}} | |||
==Biography== | ==Biography== | ||
The Faraday family came from the North of England; before Michael Faraday was born, his father, a blacksmith, took his wife and two small children to the South, in search of work. The family settled briefly in Newington Butts, a borough in South London (it was then a separate village, but is now part of Southwark), where Faraday was born. The family soon moved into London itself. | The Faraday family came from the North of England; before Michael Faraday was born, his father James, a blacksmith, took his wife Margaret and two small children to the South, in search of work. The family settled briefly in Newington Butts, a borough in South London (it was then a separate village, but is now part of Southwark), where Faraday was born. The family, which eventually included four children (two boys and two girls), soon moved into London itself, | ||
living over a stables. | |||
His father was in poor health (he died in 1810), and Faraday grew up in poverty. The family was close, and obtained strength from their orthodox faith, the[[Sandemanians]], a dissident | His father was in poor health (he died in 1810), and unable to provide well for his family; as a result, Faraday grew up in poverty. The family was close, and obtained strength from their orthodox faith, the [[Sandemanians]], a small dissident spin-off from the [[Presbyterian]] church. Faraday would stay faithful to that religion for the rest of his life. Very little is known of Faraday's early life, but he apparently received only an elementary education, being taught how to read, write, and do simple arithmetic. | ||
At fourteen he became an apprentice | In 1804, at the age of thirteen, out of economic necessity he began work, as a delivery-boy for the shop of the bookseller and bookbinder George Riebau, a French émigré.<ref>Some works give the name as ''Ribeau'', but this seems to be incorrect.</ref> At fourteen he became an apprentice with Riebau, and moved in with Riebau's family. The easy familiarity with mechanical activities which he picked up in this job no doubt stood him in good stead in his later life as an experimentalist. | ||
In a bookseller's household there were always books around for him to read, and Faraday was quick to take advantage of this. For instance, the third edition of the ''[[Encyclopaedia Britannica]]'' was one of the shop's bookbinding assignments, and his fascination for electricity was first stirred by reading the encyclopedia article about it. His first simple experiments at this time, which included a crude [[electrostatic]] generator and a weak [[voltaic pile]], were performed as a result of reading it. | |||
From 1810, encouraged by Riebau, Faraday began to attend well-attended public lectures given by [[John Tatum]] which covered the entire range of 'natural philosophy', as science was then known; his older brother Robert paid his entrance fees. These covered a number of different topics, but those on electricity, [[galvanism]] and mechanics were of particular interest to Faraday. He made detailed notes of Tatum's lectures, which he later bound into a set of four volumes, and presented to Riebau, inscribed with a dedication thanking him for his encouragement of Faraday's interests in the sciences. <ref>Hamilton, James ''A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution'' Random House, New York, (2004), pp. 10-12.</ref> At this time, Faraday also joined the City Philosophical Society, which had been founded in 1808, and consisted of a number of (youngish) people devoted to self-improvement, who met every other week at Tatum's house to hear and give lectures on scientific topics and to discuss them.<ref>Hamilton, James ''A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution'', pg. 12.</ref> It was there where Faraday delivered his very first lecture. | |||
===Initial professional career=== | ===Initial professional career=== | ||
At twenty-one, nearing the end of his apprenticeship, he | At twenty-one, nearing the end of his apprenticeship, he was given tickets for a series of four lectures on chemistry delivered by Sir [[Humphrey Davy]] at the [[Royal Institution]], a gift from a customer of the bookshop,<!--It was Dance jr. who was the customer of Riebau and it was Dance sr. who was the member of the RI, so strictly this is not correct.--> who was a member of the Royal Institution. These lectures, in the spring of 1812, were recorded by Faraday in careful lecture notes, which he neatly bound into a book. Faraday later sent a copy of his lecture notes to Davy, who was no doubt pleased by the attention to his lectures; he interviewed Faraday, but at that point could do nothing for him.<ref>[http://www.rigb.org/heritage/faradaypage.jsp Faraday Heritage], Royal Institution</ref> | ||
By the fall of 1812 Faraday was a fully-qualified bookbinder, and moved to another firm. He did not particularly like his new job, but within a few weeks his life took a sudden turn. Davy needed help for a few days at the Royal Institution, from someone with a rudimentary knowledge of chemistry, and Faraday got the job (probably as the result of a recommendation from a customer of his first employer). After the temporary job ended, Faraday had to leave the Royal Institution. However, as luck would have it, soon afterwards Davy's laboratory assistant was fired after becoming involved in a fight at the Royal Institution (apparently because of a drinking problem), and the position became vacant. Not surprisingly, Faraday got the job, and started work as Chemical Assistant at the Royal Institution on March 1, 1813, where he would stay for fifty-two years, until 1865. | |||
Half a year later, Faraday was invited by Davy to accompany Lady Davy and him on a tour of continental [[Europe]]. Davy was a renowned "natural philosopher" (the name "scientist" for this profession was only coined later, by [[William Whewell]]), and had unquestioned entry to scientific circles there. Faraday accepted the invitation, but during the tour sometimes regretted that decision, because Lady Davy (who is known to have been very snobbish) treated him as a lowly servant. Professionally, however, the tour was a great success for Faraday, because he had the chance to converse with many of the leading scientists in France, Switzerland and Italy, including such figures as [[André-Marie Ampère|Ampère]] and [[Alessandro Volta|Volta]]. In addition, he saw the [[Alps]] and the [[Mediterranean]], learned [[French (language)|French]] and [[Italian (language)|Italian]], and became Davy's collaborator, not just his assistant. | |||
The chemist [[John Hall Gladstone]] (1827–1902), who knew Faraday well, wrote: | |||
<blockquote> | |||
''This year and a half may be considered as the time of Faraday's education; it was the period of his life that best corresponds with the collegiate course of other men who have attained high distinction in the world of thought. But his University was Europe; his professors the master whom he served, and those illustrious men to whom the renown of Davy introduced the travelers.'' <ref>J.H. Gladstone, ''Michael Faraday'', (3rd ed, 1874). [http://books.google.com/books?id=Rx0AAAAAQAAJ&printsec=frontcover&dq=intitle:faraday+inauthor:gladstone&lr=&num=30&as_brr=0 online edition] </ref> | |||
</blockquote> | |||
Faraday's improved abilities were recognized upon his return to the Royal Institution; he was promoted to superintendent of apparatus, and was given better rooms (he was living at the top of the Royal Institution building in Albemarle Street). For most of the 1810s and 1820s, his direct supervisor was Davy's replacement as Professor of Chemistry, [[William Thomas Brande]]. | |||
Until early in 1820, Faraday's work was mostly in chemistry, and he earned an international reputation as a good, solid chemist—but not yet a brilliant one. His reputation as a chemist received a significant boost when he managed to produce the first known compounds of carbon and chlorine in 1820. He produced these by substituting chlorine for hydrogen in what was called 'olefiant gas' ([[ethylene]]), these were the first chemical [[substitution reaction]]s demonstrated. During the early 1820s he also investigated [[steel]] [[alloy]]s, work which helped to lay the foundations of metallurgy and metallography.<ref name="Hebrew"> [http://chem.ch.huji.ac.il/history/faraday.htm Faraday biography], Institute of Chemistry at the Hebrew University of Jerusalem. </ref> | |||
===First major discovery=== | |||
He work at the Institute had initially been almost entirely on chemistry, but electricity had always been one of his interests. In 1820, several significant discoveries on the Continent (notably by Oersted, [[Jean-Baptiste Biot|Biot]] and Ampère) which began to establish a connection between electricity and magnetism interested Davy; Faraday was therefore able to begin work in the area. His first major achievement was to show that electricity could be used to force a [[magnet]] into continual rotational motion, as long as the electricity continued to flow.<ref>According to [http://repo-nt.tcc.virginia.edu/book/chap1/chapter1sec3.htm Discovery as Invention: Michael Faraday], this was theorized before by others, but he was the first to actually demonstrate it. </ref> He also decided that if an [[electric current]] in a wire could move a magnet, then if the magnet were held fixed, and the wire allowed to be mobile, then a current flowing in the wire should cause it to move. | |||
In the experiment he created to investigate these concepts, he managed to demonstrate both of these effects (''see the section [[#Electromagnetic rotation|below]] on electromagnetic rotation for more details''). In one part of the experiment a steady [[direct current]] moved a magnet in circles; in another, a similar current caused a wire to steadily rotate. These discoveries, made in September, 1821, are the basis of all electric motors, and brought Faraday instant world fame. | |||
Unfortunately, the achievement started a rift between Davy and Faraday, which a later incident over work on liquifying chlorine was to exacerbate. Davy was apparently of the opinion that Faraday had relied on some work done by Davy and [[William Wollaston]], without properly acknowledging their contribution. (In April 1821, Wollaston, after hearing of Oersted's discovery, had visited the Royal Institution, and in collaboration with Davy had tried—in vain—something similar to what Faraday managed to do later that year.)<ref name="James"> Frank A. J. L. James, "Faraday, Michael (1791–1867)", ''Oxford Dictionary of National Biography,'' Sept 2004; online edn, Jan 2008 </ref> | |||
Also in 1821, Faraday received his first promotion at the Royal Institute (to Superintendent of the House), and married Sarah Barnard, another Sandemanian, on June 12; they never had children. Sarah was a steadying influence in Faraday's life; she was a warm and charming person filled with maternal feelings which, in the absence of children, she lavished upon her husband and her nieces. The couple lived "above the store", at the top of the Royal Institution building, until 1862. | |||
===Further scientific accomplishments=== | |||
Following a suggestion of Davy, Faraday managed to liquify chlorine in March 1823 (but only after he was lucky to escape serious injury in several unexpected laboratory explosions).<ref>Hamilton, James ''A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution'', pp. 186-188.</ref> | |||
This achievement further displeased Davy, who felt that he deserved partial credit for this discovery, since he had suggested the problem to Faraday.<ref name="James"/> Faraday also succeeded in liquifying a number of other gasses, including [[carbon dioxide]] and [[sulphur dioxide]]. | |||
Faraday became a [[Fellow of the Royal Society]] in 1824, with one vote against him; it is believed that this was Davy's. The usual explanation was that this resulted from their prior disputes, but it may have been simply because of Davy's public stance against nepotism.<ref name="Farndon">Farndon, John et al. ''The Great Scientists: From Euclid to Stephen Hawking'', Metro Books, New York (2007) pg. 82 </ref> Faraday never let Davy's opposition affect his respect for Davy, although he acknowledged that their relationship had become strained. | |||
Possibly as a result of his disputes with Davy, he was directed to spend much of the 1820s working on less important problems, but he still managed a few significant discoveries. In 1825, during research on illuminating gasses, he discovered [[benzene]], which he called ''bicarburet of hydrogen''; he isolated it from a liquid obtained in the production of oil gas. <!-- Q: What's 'oil gas', anyone know? Is it a component or byproduct of natural gas? A: It is a byproduct of crude oil, I would guess. It escapes when you heat crude oil. --> Some decades later, benzene would be one of the keys in the development of [[organic chemistry]]. | |||
During the late 1820s, he was also directed into research on glass, intended for producing better optical glass for telescopes; this did not have much result, although he created the recipe for ''heavy glass'', with a very high refractive index. | |||
In 1825, Faraday became Director of the Laboratory of the Royal Institution and, beginning in 1826, he revived the tradition of popular lectures at the Institution, giving many himself. For many years, around Christmas he and others delivered a short lecture series especially for children, which attracted an audience from the upper social classes of London. The most famous of the Christmas lecture series, from 1848, called ''The Chemical History of a Candle'', was published, and has since gone through innumerable editions in many languages.<ref>M. Faraday, ''The Chemical History Of A Candle'' (1908) [http://books.google.com/books?id=Qb-CKerc6J0C&pg=PA1&dq=inauthor:Faraday+inauthor:Michael&lr=&num=30&as_brr=0&sig=xU_34b5hgcWe6WNBTAseQLlWnWc online edition]</ref> Another well-known series directed to young persons is about various forces in nature, from 1859.<ref>M. Faraday, ''On the Various Forces in Nature and their relations to each other: a course of lectures delivered before a juvenile audience at the Royal Institution'', (six lectures)[http://ia340905.us.archive.org/3/items/onvariousforceso00farauoft/onvariousforceso00farauoft_bw.pdf online] </ref> These Christmas lectures continue to this day, and are now televised, thereby reaching a much larger audience than the originals. | |||
===Most significant scientific achievement=== | |||
After Davy's death in 1829, Faraday moved back to important areas of research, returning an area which he had first investigated in December 1824, which was the use of a magnet to produce electricity. (At this point in time, the only ways to produce electricity were with rubbing glass and amber <!-- American's first electrician , Benjamin Franklin, never knew of batteries-->and—since Volta's discovery of 1800—by primitive chemical [[batteries]].) In August, 1831 he made what some consider his most important discovery, which is that a changing [[magnetic field]] (either from a moving magnet, or a wire moving through a magnetic field) can 'induce' an electric current in a wire (''see the section [[#Electromagnetic induction|below]] on electromagnetic induction for more details''). He named this phenomenon ''[[electromagnetic induction]]'', and it is used today in almost all production of electricity, as well as [[AC motors]]. | |||
In 1833 Faraday was honored by being appointed [[Fullerian Professor]] of Chemistry at the Royal Institution; the chair was especially created for him, and still exists today. (One of Faraday's biographers, J. H. Gladstone, later held the Fullerian professorship for three years.) He was to receive a number of other honours over the years, such as the Royal Medal and the [[Copley Medal]] (both from the [[Royal Society]]). | |||
Faraday | Starting in 1832 Faraday also began an investigation which fortuitously turned into the very important work he did in electrochemistry. He started out with a desire to show that the various forms of electricity ([[static electricity]], electricity produced by a battery, electricity in biology, e.g., of [[electric rays]], and electricity produced by his induction methods) were all the same thing. In the course of this work, he discovered that it was the actual passage of electricity through a conducting liquid which decomposed the chemicals therein, not some sort of ''action at a distance'' of electricity, as had previously been believed. His extended investigations in this area laid the groundwork for electrochemistry, and he formulated two laws in that area which now carry his name. He also devised the terminology used in this field, which was derived from classical [[Greek (language)|Greek]], with the assistance of his friend William Whewell of [[Trinity College]], Cambridge, who knew the language. | ||
===Fields=== | |||
During this entire period, and continuing on through the 1840s, he was developing the idea of electric and magnetic ''force lines''. Because of his background, Faraday knew hardly any mathematics, and his intuitive ideas were qualitative and non-mathematical, so he was not able to put them into formal terms. Some details of his concepts contradicted the then widely-held belief that electromagnetic effects involved ''instantaneous action at a distance''. Most of the contemporary physicists, who generally were well versed in the mathematical formulation of Sir [[Isaac Newton]]'s mechanics, in which instantaneous action at a distance plays an important role, frowned upon Faraday's ideas. | |||
<!-- Q: I think I have put this correctly. I had a quick look for something that talks about Faraday's views on 'instantanous', but didn't quickly find anything. | |||
A: An essential property of fields is that they build up in finite time, Faraday was well aware of this. He asked the question: if the Earth is suddenly removed how can the Sun know this instantaneously? Answer is: it cannot, it takes time before the change in gravitational field reaches the Sun. --> | |||
They looked upon electricity as an immaterial fluid that flows through matter; Faraday took a different point of view. He thought of it as a vibration, which was transmitted from place to place by intermediate contiguous particles. (Later physicists revived [[Descartes]]' idea of the [[ether (electromagnetism)|ether]] to carry the vibrations.) | |||
The | By 1850, Faraday's thinking had produced a radically new view of space: instead of being 'nothingness', a mere void in which were located various material objects. Rather, he saw it as a medium capable of supporting electric and magnetic forces, through collections of what he called ''lines of force''. The forces were not localized in the particles which were the source of them; rather, their manifestation, the force lines, were to be found throughout the space around them. This marked the birth of field theory, which today is a key concept in all of physics. The collection of all the force lines forms a ''field of force'', a term coined by [[William Thomson]] (later Lord Kelvin), who advocated and extended Faraday's ideas; initially, Faraday felt that Thomson was the only scientist who really understood his field ideas.<ref name="Farndon" /> | ||
< | |||
' | Thomson's ideas were later taken up and extended and refined by [[James Clerk Maxwell]], who maintained that the basic ideas for his mathematical theory of electromagnetic fields came from Faraday, and that his contribution was to turn them into a concise and elegant mathematical form.<ref name="Hebrew"/> Maxwell's characterization of his contributions, while admittedly first-hand, may be overly modest. 'Maxwell's equations', as they are now known, are today still the accepted form of the theory of electromagnetism. Among many other important results, they show that visible [[light]] consists of [[electromagnetic waves]], as are [[radio waves]], [[microwaves]], [[infrared radiation|infrared]] and [[gamma radiation]], which are collectively known as [[electromagnetic radiation]]. | ||
===Last major discovery=== | |||
Faraday was strong physically, but suffered occasionally from headaches, memory lapses, and bouts of depression. These symptoms increased in severity and frequency until in 1840, at the age of forty-nine, Faraday had a major breakdown, the exact nature of which is not certain. For four years he was hardly able to work, and his health never fully recovered. | |||
However, by 1845 he was well enough to resume work, and he started his research activities again. Because he believed strongly in the unity of forces, he again investigated the effect of magnetic fields on light, an area he had previously investigated starting in 1822, but without success; in September, 1845, he made another major discovery. Acting on a suggestion by William Thomson, whose mathematical work on the Faraday's field ideas had produced a prediction that a magnetic field should affect [[polarized light]],<ref> [http://www-groups.dcs.st-and.ac.uk/~history/Biographies/Faraday.html Faraday biography], School of Mathematical and Computational Sciences at the University of St Andrews </ref> he discovered such a connection; the [[polarization plane]] of polarized light is rotated by a magnetic field. This ability of magnetism to affect light is now known as the [[Faraday effect]]. | |||
In | In the same series of experiments, he also discovered [[diamagnetism]]. Although these results did not have such important direct practical applications as some of his earlier work, there were of considerable importance in the development of electromagnetic theory. | ||
===Later life=== | |||
True to his Sandemanian principles, and his indifference to honours and fame, Faraday turned down the offer of a [[knighthood]], and twice declined to become president of the Royal Society. In 1861, when he was seventy years old, he resigned from the Royal Institution, but he was asked to stay on in a nominal post, which he did until 1865. In 1862, he and Sarah moved out from Albemarle Street into a house at [[Hampton Court]] provided a few years earlier by [[Queen Victoria]], at the suggestion of her husband [[Prince Albert]]. He died there on August 25, 1867. In a characteristic display of his lifelong modesty, he had turned down an offer to be buried in [[Westminster Abbey]], as he preferred a simpler funeral and grave (although he does have a memorial plaque there); he is buried in [[Highgate Cemetery]]. | |||
[[Image:Faraday20PoundNote.jpg|right|300px]] | |||
Faraday | Faraday is memorialized in a number of ways: in addition to the [[farad]], an electrical unit named after him, statues of him stand at the Royal Institution, and outside the [[Institution of Electrical Engineers]] in London; a number of university buildings also bear his name. In perhaps his most notable honour, his image appeared on the British 20 pound banknote (see image at right) for some years—an honour given to only a very few scientists. | ||
His successor in giving popular lectures on science at the Royal Institute, [[John Tyndall]], who in 1853 became a professor at the Royal Institution, said of him: | |||
<blockquote> | |||
''Taking him for all and all, I think it will be conceded that Michael Faraday was the greatest experimental philosopher the world has ever seen; and I will add the opinion, that the progress of future research will tend, not to dim or to diminish, but to enhance and glorify the labours of this mighty investigator.'' | |||
</blockquote> | |||
Time has fully confirmed the accuracy of Tyndall's estimation. | |||
==Faraday's science== | ==Faraday's science== | ||
This section contains some more technical detail on his most important results in physics and chemistry. | |||
===Electromagnetic rotation=== | ===Electromagnetic rotation=== | ||
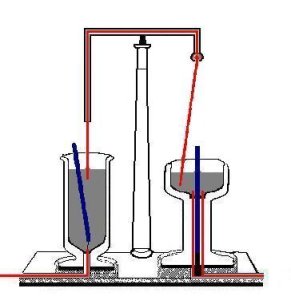
{{Image|Faraday electromagnetic rotation.jpg|right|350px|Adaptation of Faraday's illustration of his apparatus for electromagnetic rotation. The vessels are filled with mercury (grey), current runs through copper wires (red) and the bar magnets, floating on the left, and fixed on the right, are blue.}} | |||
When Faraday heard of Oersted's 1820 discovery that a steady electric current in a wire generates a cylindrical magnetic field (with the current-carrying wire as the axis of the cylinder), it occurred to him that a [[magnetic pole]] would be pushed around a circle by such a field. Hence, it would rotate forever, or at least as long as the current is flowing. He also reasoned that if a current in a wire can move a magnet, a magnet should be able to move a current-carrying wire. In 1821 he designed the apparatus shown in the figure on the right: it includes two distinct mechanisms, one for each of the two basic concepts he was working on. In one, electricity propels a moveable magnet, and in the other, a fixed magnet causes a mobile wire to move when electricity flows through it.<ref> A modification of Plate IV in: M. Faraday, ''Experimental researches in electricity'', vol II, Richard and John Edward Taylor, London (1844). [http://gallica.bnf.fr/ark:/12148/bpt6k94884v/f3.pagination On line] </ref> | |||
In the vessel on the left, a strong bar magnet floats on end in a [[Mercury (element)|mercury]] bath, held in place only by a thread at its bottom. (Recall that mercury is a heavy, liquid, and metallic element that is a very good conductor of electricity.) A fixed [[copper]] wire dips into the mercury at the top of the bath; at the bottom of the vessel, another wire also projects into the mercury. | |||
In the vessel on the right, another bar magnet is fixed in an upright position in the middle of another bath of mercury. A conducting (copper) socket extends into the bottom of the bath, and a copper wire which hangs from a flexible joint above the mercury bath dips into the top of the bath; the joint allows the top wire to pivot relatively freely around the joint. | |||
When | When a direct current is switched on in either vessel (running in through one wire, through the mercury bath, and out through the other wire), it produces motion of the mechanism in that bath. The wire in the one on the right rotates around the magnet so fast that—as described by Faraday—the eye can scarcely follow the motion; the magnet on the left rotates around the fixed wire. | ||
Note that Faraday's setup is such that only one pole of each of the two poles of the magnets is employed. If either of the magnets is turned around (i.e., the North and South poles are interchanged), the rotation which is observed in that vessel changes direction. The same happens if the direction of the current is reversed. Faraday, who coined the term ''electromagnetic rotation'' for this effect, had in fact invented a primitive precursor to the electric motor. | |||
===Electromagnetic induction=== | ===Electromagnetic induction=== | ||
[[Image:Faraday ring.jpg|left|thumb|Faraday's equipment with which he discovered electromagnetic induction | [[Image:Faraday ring.jpg|left|thumb|Faraday's equipment with which he discovered electromagnetic induction.<ref> Bence Jones, ''The Life and Letters of Faraday'' vol. II, [http://books.google.com/books?id=9C0DAAAAYAAJ&printsec=frontcover&dq=inauthor:Michael+inauthor:Faraday&lr=lang_en&num=100&as_brr=0&hl=nl online]</ref>]] | ||
In a series of experiments performed in August, 1831, Faraday, knowing that electricity can create magnetism, investigated whether the converse effect is also true; in other words, whether magnetism has an effect on an electric current. | |||
For this, he wound two coils of insulated wire around either side of a soft iron ring of six inches external diameter; the ring itself was 7/8 inch thick. (See the illustration on the left; the original apparatus for this, and other of his induction experiments, is preserved to this day in the Royal Institution.) The coils were not connected to each other. The coil ''A'' on the right-hand side could be connected to a battery; a copper wire attached to the coil ''B'' was led over a magnetic needle a few feet away from the ring. At the moment the battery was connected to coil ''A'', and the current began to flow, the needle oscillated, and after a while settled into its original position. When the battery was disconnected from ''A'', a disturbance of the needle was again observed—a result which surprised Faraday, who had not expected to see a pulse from both stopping as well as starting the flow of electricity. | |||
When Faraday performed this experiment, it was already known that the part of the iron ring covered by coil ''A'' becomes a magnet—an [[electromagnet]]—when current runs through the coil. (The positioning of the test magnetic needle a few feet away was necessary to ensure that the electromagnetism of the powered coil ''A'' did not affect the magnetic needle.) The magnetic North and South poles of this electromagnet are at the beginning and the end of coil ''A''—which end is North depends on the direction of the current in the coil ''A''. The [[magnetic field]] produced by the electromagnet is effectively channeled from one end of it around to the other end through the iron of the ring, which is more hospitable to magnetic fields than air, a property known as [[magnetic permeability]], thereby turning the entire ring into a magnet. | |||
Since the iron ring passes through coil ''B'', then as long as the current is flowing through coil ''A'', ''B'' is wound around a magnet as well. Faraday had discovered that changes in the strength of the magnetic field of the electromagnet (which occur when switching it on and off) produces a current in the wire connected to coil ''B'' (which, remember, is not in electric contact with ''A''). The proof that a current was running through ''B'' was the movement of the magnetic needle; it was known from Oersted's work that a current flowing in a wire will move a nearby magnetic needle. | |||
Within a few weeks he further investigated this effect by setting up a cylindrical coil of about 4 centimeters diameter and about 16 centimeters long, made with 70 meters of wire. He then rapidly moved a permanent cylindrical bar magnet of about 22 centimeters length up and down inside the coil. He then was able to observe an electric current produced in the coil, 'induced' by the moving magnet. The direction of the current produced in the coil when the magnet was moved into the coil was the reverse of that produced when it was then pulled out. This important experiment proved that moving a wire through a magnetic field produced a current in the wire (because it was equivalent to holding the magnet still, and moving the coil instead). | |||
He later succeeding in generating a current by rotating a copper disk between the poles of a large horseshoe magnet; the disk had one wire fixed to its center, and another made sliding contact along the edge of the disk. This was the first generator, and all generators today are its descendants. It also pointed the way toward the realization of an electric motor, since reversing the operation (by feeding an electric current into the disk) would make it rotate. | |||
Faraday called this effect electromagnetic induction, and he was fascinated by the symmetry revealed by the effect that he now had discovered. Previously, it had been known that a moving electric charge (i.e. a current) produced a magnetic field; now it had been shown that a moving magnet produced an electric field (it is this field which causes the current to flow). Magnetic induction is of extreme importance in modern industrial society, because it is the principle behind [[electric generator]]s and [[electric transformer|transformers]]. | |||
<!-- (The importance of the latter is that without simple, passive device to alter the [[voltage]] of electricity, economical and safe distribution of electric power would not have been possible.) | |||
I would save this statement for an article on transformers (PW). | |||
Well, I put it in because I thought it would help our average readers understand the importance of this discovery. But I don't feel strongly that it has to be here. [jnc] | |||
--> | |||
===Electrochemistry=== | |||
The basic concept behind electrochemistry is quite simple: it consists of a vessel containing an [[electrolyte]], which is a solution of charged particles ([[ion]]s). A direct electric current is run through the electrolyte, introduced via [[electrode]]s which are dipped into it; the ensuing decomposition of chemicals in the solution is known as [[electrolysis]]. Depending on the electrolyte's chemical composition, and the makeup of the electrodes, a large range of useful chemical reactions result at the electrodes. | |||
Since the solution is electrically neutral overall, the total charge carried by the positive particles ([[cations]]) is the same (in absolute value) as the total charge carried by the negative particles ([[anions]]). The current runs from the negatively charged electrode (the ''cathode'') to the positively charged electrode (the ''anode''). Inside the vessel, cations move to the cathode, pick up [[electrons]]—which carry negative charge—so that the cations are neutralized, and are deposited on the cathode. If the neutral product is gaseous, it escapes from the cathode in the form of gas. At the anode, the opposite happens: anions lose their excess electrons, and are deposited on the anode. Outside the vessel, the electrons run from the anode to the cathode. | |||
Faraday's first law of electrochemistry states that the amount of substance deposited on the electrodes is proportional to the total amount of current that has passed through the electrodes. In the case of a steady current, this is equal to the time that the current has been running, multiplied by the [[amperage]] (flow rate) of the current. A constant proportional to this amount is named after him—[[Faraday's constant]]. | |||
Faraday's second law of electrochemistry is a recognition of the fact that anions and cations may carry more than one [[elementary charge]]s (although their number is always a small integer). Therefore, it takes a corresponding number of electrons to neutralize the cation, while for the anion to become neutral, it must lose a like number of electrons. These differently charged ions will all produce the deposition of only one atom from the requisite number of electrons, so that twice as much electricity is needed to generate an atom from a doubly charged ion as from a singly charged ion. | |||
Faraday was the first to see these relationships clearly; moreover, all the terminology in the field (electrolysis, electrolyte, electrode, anode, cathode, ion, anion, and cation) was created by him. | |||
=== | ===Dielectrics and Faraday's cage=== | ||
In | In his work on electrolysis, Faraday had noticed that many liquids which conduct an electric current become non-conducting when frozen. For instance, slightly acidic water is a good conductor, but when turned into ice is an insulator. Moreover, two plate electrodes with opposite electrical charge on them attract each other, even if a non-conducting substance—called by Faraday a ''[[dielectric]]''—is in between the plates. | ||
For Faraday this meant that the electrostatic attraction which produced that attractive force is not an action at a distance—as was generally assumed at the time—but a force conveyed by contiguous particles. <!-- How did he reach that conclusion from that starting point? And what's a 'contiguous particle'? | |||
If it had been action at a distance, liquid or solid wouldn't have made a difference. "Contiguous" is a term used by Faraday himself. I take it that it means something like a consecutive, continuous row of particles, connecting one electrode with another. --> In the second half of the 1830s he started a research program intended to prove these ideas about lines of force which are carried by intermediate particles (a concept later becoming important as [[ether (electromagnetism)|ether]]), trying to disprove instantaneous action at a distance. | |||
In the course of this research program, in November 1837, Faraday had a wooden cube | To prove his point about the nature of electrostatic forces, Faraday constructed [[capacitor]]s of different shapes and sizes, and experimented with all kinds of dielectrics. To honor his work in this area, the [[SI]] unit of [[capacitance]] (the amount of charge that can be stored in a capacitor) is called a ''farad''. | ||
In the course of this research program, in November 1837, Faraday had a large wooden cube built, big enough to hold a person and some scientific apparatus, and had the sides completely covered with a network of conducting wires. He gives the following description of it: | |||
<blockquote> | |||
''I went into the cube and lived in it, and using lighted candles, electrometers, and all other tests of electrical states, I could not find the least influence upon them, or indication of anything particular given by them, though all the time the outside of the cube was powerfully charged, and large sparks and brushes were darting off from every part of its outer surface.'' <ref>M. Faraday, ''Experimental researches in electricity'', vol I, 2nd edition, | |||
Richard and John Edward Taylor, London (1849). p. 366 [http://gallica.bnf.fr/ark:/12148/CadresFenetre?O=NUMM-94883&M=pagination On line] </ref> | Richard and John Edward Taylor, London (1849). p. 366 [http://gallica.bnf.fr/ark:/12148/CadresFenetre?O=NUMM-94883&M=pagination On line] </ref> | ||
</blockquote> | </blockquote> | ||
The results of his experiments in the cube enabled him show that electricity was a force rather than an imponderable fluid, as was argued by some physicists at that time. | The results of his experiments in the cube enabled him show that electricity was in fact a force rather than an imponderable fluid, as was argued by some physicists at that time. | ||
We would now call the conducting cube he constructed a ''Faraday cage''; they are notorious among people trying to use cellular phones in buildings, and are a life-saver for car occupants in thunderstorms. | |||
== | ==Further reading== | ||
* Cantor, Geoffrey N. ''Michael Faraday: Sandemanian and Scientist'' Macmillan (1991) - Explores the potential connections between Faraday's religious beliefs and his science. | |||
* Cantor, G. N., David Gooding, and Frank A. J. L. James ''Michael Faraday'' Humanity Books, New York (1996) - A slim paperback, it still provides a good overview of Faraday as a person (including his religious beliefs), his scientific career and discoveries, and his influence; it also contains an interesting, brief, historiographical note. | |||
* Cantor, Geoffrey. ''Michael Faraday: Sandemanian and Scientist | * Hamilton, James ''A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution'' Random House, New York, (2004) ISBN 1-4000-6016-8 - Contains extensive quotations from original documents, and is well footnoted, but focuses more on Faraday the person than the details of his scientific work. | ||
* Cantor, | * Hirshfeld, Alan W. ''The Electric Life of Michael Faraday'' Walker (2006) - A modestly-sized volume that is more weighted toward the actual science than the Hamilton volume; includes an interesting chapter on the relationship between Faraday and Maxwell. | ||
* | * James, Frank A. J. L. "Faraday, Michael (1791–1867)", ''Oxford Dictionary of National Biography'', Sept 2004; online edn, Jan 2008 | ||
* Williams, L. Pearce ''Michael Faraday, A Biography'' Basic Books, New York (1965) - The first modern scholarly biography, this lengthy work covers his scientific career in considerable details, and contains extensive quotations from Faraday's original writings; each chapter ends with extensive source notes. | |||
* | |||
* James, Frank A. J. L. "Faraday, Michael (1791–1867)", ''Oxford Dictionary of National Biography | |||
* Williams, L. Pearce | |||
====Notes==== | ====Notes==== | ||
<references /> | <references />[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:00, 18 September 2024
Michael Faraday (September 22, 1791 – August 25, 1867) was an English physicist and chemist who is one of the most influential scientists of all time.[1] His most important contributions, and best known work, were on the closely connected phenomena of electricity and magnetism, but he also made very significant contributions in chemistry.
Faraday was principally an experimentalist; in fact, he has been described as the "best experimentalist in the history of science".[2] He did not know any advanced mathematics, however.[3] Both his contributions to science, and his impact on the world, are nonetheless vast: his scientific discoveries underlie significant areas of modern physics and chemistry, and the technology which evolved from his work is even more widespread. His discoveries in electromagnetism laid the groundwork for the engineering work in the late 1800s by people such as Edison, Siemens, Tesla and Westinghouse, which brought about the electrification of industrial societies, and his work in electrochemistry is now widely used in the field of chemical engineering.
In physics, he was one of the first to explore the ways in which electricity and magnetism are connected. In 1821, shortly after Oersted first discovered that electricity and magnetism were associated, Faraday published his work on what he called electromagnetic rotation (the principle behind the electric motor). In 1831, Faraday discovered electromagnetic induction, the principle behind the electric generator and electric transformer. His ideas about electrical and magnetic fields, and the nature of fields in general, inspired later work in this area (such as Maxwell's equations), and fields of the type he envisaged are a key concept in today's physics.
In chemistry, he created the first known compounds of carbon and chlorine, helped to lay the foundations of metallurgy and metallography, succeeded in liquifying a number of gasses for the first time, and discovered benzene. Perhaps his biggest contribution was in virtually founding electrochemistry, and introducing terminology such as electrolyte, anode, cathode, electrode, and ion. [4]
Biography
The Faraday family came from the North of England; before Michael Faraday was born, his father James, a blacksmith, took his wife Margaret and two small children to the South, in search of work. The family settled briefly in Newington Butts, a borough in South London (it was then a separate village, but is now part of Southwark), where Faraday was born. The family, which eventually included four children (two boys and two girls), soon moved into London itself, living over a stables.
His father was in poor health (he died in 1810), and unable to provide well for his family; as a result, Faraday grew up in poverty. The family was close, and obtained strength from their orthodox faith, the Sandemanians, a small dissident spin-off from the Presbyterian church. Faraday would stay faithful to that religion for the rest of his life. Very little is known of Faraday's early life, but he apparently received only an elementary education, being taught how to read, write, and do simple arithmetic.
In 1804, at the age of thirteen, out of economic necessity he began work, as a delivery-boy for the shop of the bookseller and bookbinder George Riebau, a French émigré.[5] At fourteen he became an apprentice with Riebau, and moved in with Riebau's family. The easy familiarity with mechanical activities which he picked up in this job no doubt stood him in good stead in his later life as an experimentalist. In a bookseller's household there were always books around for him to read, and Faraday was quick to take advantage of this. For instance, the third edition of the Encyclopaedia Britannica was one of the shop's bookbinding assignments, and his fascination for electricity was first stirred by reading the encyclopedia article about it. His first simple experiments at this time, which included a crude electrostatic generator and a weak voltaic pile, were performed as a result of reading it.
From 1810, encouraged by Riebau, Faraday began to attend well-attended public lectures given by John Tatum which covered the entire range of 'natural philosophy', as science was then known; his older brother Robert paid his entrance fees. These covered a number of different topics, but those on electricity, galvanism and mechanics were of particular interest to Faraday. He made detailed notes of Tatum's lectures, which he later bound into a set of four volumes, and presented to Riebau, inscribed with a dedication thanking him for his encouragement of Faraday's interests in the sciences. [6] At this time, Faraday also joined the City Philosophical Society, which had been founded in 1808, and consisted of a number of (youngish) people devoted to self-improvement, who met every other week at Tatum's house to hear and give lectures on scientific topics and to discuss them.[7] It was there where Faraday delivered his very first lecture.
Initial professional career
At twenty-one, nearing the end of his apprenticeship, he was given tickets for a series of four lectures on chemistry delivered by Sir Humphrey Davy at the Royal Institution, a gift from a customer of the bookshop, who was a member of the Royal Institution. These lectures, in the spring of 1812, were recorded by Faraday in careful lecture notes, which he neatly bound into a book. Faraday later sent a copy of his lecture notes to Davy, who was no doubt pleased by the attention to his lectures; he interviewed Faraday, but at that point could do nothing for him.[8]
By the fall of 1812 Faraday was a fully-qualified bookbinder, and moved to another firm. He did not particularly like his new job, but within a few weeks his life took a sudden turn. Davy needed help for a few days at the Royal Institution, from someone with a rudimentary knowledge of chemistry, and Faraday got the job (probably as the result of a recommendation from a customer of his first employer). After the temporary job ended, Faraday had to leave the Royal Institution. However, as luck would have it, soon afterwards Davy's laboratory assistant was fired after becoming involved in a fight at the Royal Institution (apparently because of a drinking problem), and the position became vacant. Not surprisingly, Faraday got the job, and started work as Chemical Assistant at the Royal Institution on March 1, 1813, where he would stay for fifty-two years, until 1865.
Half a year later, Faraday was invited by Davy to accompany Lady Davy and him on a tour of continental Europe. Davy was a renowned "natural philosopher" (the name "scientist" for this profession was only coined later, by William Whewell), and had unquestioned entry to scientific circles there. Faraday accepted the invitation, but during the tour sometimes regretted that decision, because Lady Davy (who is known to have been very snobbish) treated him as a lowly servant. Professionally, however, the tour was a great success for Faraday, because he had the chance to converse with many of the leading scientists in France, Switzerland and Italy, including such figures as Ampère and Volta. In addition, he saw the Alps and the Mediterranean, learned French and Italian, and became Davy's collaborator, not just his assistant.
The chemist John Hall Gladstone (1827–1902), who knew Faraday well, wrote:
This year and a half may be considered as the time of Faraday's education; it was the period of his life that best corresponds with the collegiate course of other men who have attained high distinction in the world of thought. But his University was Europe; his professors the master whom he served, and those illustrious men to whom the renown of Davy introduced the travelers. [9]
Faraday's improved abilities were recognized upon his return to the Royal Institution; he was promoted to superintendent of apparatus, and was given better rooms (he was living at the top of the Royal Institution building in Albemarle Street). For most of the 1810s and 1820s, his direct supervisor was Davy's replacement as Professor of Chemistry, William Thomas Brande.
Until early in 1820, Faraday's work was mostly in chemistry, and he earned an international reputation as a good, solid chemist—but not yet a brilliant one. His reputation as a chemist received a significant boost when he managed to produce the first known compounds of carbon and chlorine in 1820. He produced these by substituting chlorine for hydrogen in what was called 'olefiant gas' (ethylene), these were the first chemical substitution reactions demonstrated. During the early 1820s he also investigated steel alloys, work which helped to lay the foundations of metallurgy and metallography.[10]
First major discovery
He work at the Institute had initially been almost entirely on chemistry, but electricity had always been one of his interests. In 1820, several significant discoveries on the Continent (notably by Oersted, Biot and Ampère) which began to establish a connection between electricity and magnetism interested Davy; Faraday was therefore able to begin work in the area. His first major achievement was to show that electricity could be used to force a magnet into continual rotational motion, as long as the electricity continued to flow.[11] He also decided that if an electric current in a wire could move a magnet, then if the magnet were held fixed, and the wire allowed to be mobile, then a current flowing in the wire should cause it to move.
In the experiment he created to investigate these concepts, he managed to demonstrate both of these effects (see the section below on electromagnetic rotation for more details). In one part of the experiment a steady direct current moved a magnet in circles; in another, a similar current caused a wire to steadily rotate. These discoveries, made in September, 1821, are the basis of all electric motors, and brought Faraday instant world fame.
Unfortunately, the achievement started a rift between Davy and Faraday, which a later incident over work on liquifying chlorine was to exacerbate. Davy was apparently of the opinion that Faraday had relied on some work done by Davy and William Wollaston, without properly acknowledging their contribution. (In April 1821, Wollaston, after hearing of Oersted's discovery, had visited the Royal Institution, and in collaboration with Davy had tried—in vain—something similar to what Faraday managed to do later that year.)[12]
Also in 1821, Faraday received his first promotion at the Royal Institute (to Superintendent of the House), and married Sarah Barnard, another Sandemanian, on June 12; they never had children. Sarah was a steadying influence in Faraday's life; she was a warm and charming person filled with maternal feelings which, in the absence of children, she lavished upon her husband and her nieces. The couple lived "above the store", at the top of the Royal Institution building, until 1862.
Further scientific accomplishments
Following a suggestion of Davy, Faraday managed to liquify chlorine in March 1823 (but only after he was lucky to escape serious injury in several unexpected laboratory explosions).[13] This achievement further displeased Davy, who felt that he deserved partial credit for this discovery, since he had suggested the problem to Faraday.[12] Faraday also succeeded in liquifying a number of other gasses, including carbon dioxide and sulphur dioxide.
Faraday became a Fellow of the Royal Society in 1824, with one vote against him; it is believed that this was Davy's. The usual explanation was that this resulted from their prior disputes, but it may have been simply because of Davy's public stance against nepotism.[14] Faraday never let Davy's opposition affect his respect for Davy, although he acknowledged that their relationship had become strained.
Possibly as a result of his disputes with Davy, he was directed to spend much of the 1820s working on less important problems, but he still managed a few significant discoveries. In 1825, during research on illuminating gasses, he discovered benzene, which he called bicarburet of hydrogen; he isolated it from a liquid obtained in the production of oil gas. Some decades later, benzene would be one of the keys in the development of organic chemistry.
During the late 1820s, he was also directed into research on glass, intended for producing better optical glass for telescopes; this did not have much result, although he created the recipe for heavy glass, with a very high refractive index.
In 1825, Faraday became Director of the Laboratory of the Royal Institution and, beginning in 1826, he revived the tradition of popular lectures at the Institution, giving many himself. For many years, around Christmas he and others delivered a short lecture series especially for children, which attracted an audience from the upper social classes of London. The most famous of the Christmas lecture series, from 1848, called The Chemical History of a Candle, was published, and has since gone through innumerable editions in many languages.[15] Another well-known series directed to young persons is about various forces in nature, from 1859.[16] These Christmas lectures continue to this day, and are now televised, thereby reaching a much larger audience than the originals.
Most significant scientific achievement
After Davy's death in 1829, Faraday moved back to important areas of research, returning an area which he had first investigated in December 1824, which was the use of a magnet to produce electricity. (At this point in time, the only ways to produce electricity were with rubbing glass and amber and—since Volta's discovery of 1800—by primitive chemical batteries.) In August, 1831 he made what some consider his most important discovery, which is that a changing magnetic field (either from a moving magnet, or a wire moving through a magnetic field) can 'induce' an electric current in a wire (see the section below on electromagnetic induction for more details). He named this phenomenon electromagnetic induction, and it is used today in almost all production of electricity, as well as AC motors.
In 1833 Faraday was honored by being appointed Fullerian Professor of Chemistry at the Royal Institution; the chair was especially created for him, and still exists today. (One of Faraday's biographers, J. H. Gladstone, later held the Fullerian professorship for three years.) He was to receive a number of other honours over the years, such as the Royal Medal and the Copley Medal (both from the Royal Society).
Starting in 1832 Faraday also began an investigation which fortuitously turned into the very important work he did in electrochemistry. He started out with a desire to show that the various forms of electricity (static electricity, electricity produced by a battery, electricity in biology, e.g., of electric rays, and electricity produced by his induction methods) were all the same thing. In the course of this work, he discovered that it was the actual passage of electricity through a conducting liquid which decomposed the chemicals therein, not some sort of action at a distance of electricity, as had previously been believed. His extended investigations in this area laid the groundwork for electrochemistry, and he formulated two laws in that area which now carry his name. He also devised the terminology used in this field, which was derived from classical Greek, with the assistance of his friend William Whewell of Trinity College, Cambridge, who knew the language.
Fields
During this entire period, and continuing on through the 1840s, he was developing the idea of electric and magnetic force lines. Because of his background, Faraday knew hardly any mathematics, and his intuitive ideas were qualitative and non-mathematical, so he was not able to put them into formal terms. Some details of his concepts contradicted the then widely-held belief that electromagnetic effects involved instantaneous action at a distance. Most of the contemporary physicists, who generally were well versed in the mathematical formulation of Sir Isaac Newton's mechanics, in which instantaneous action at a distance plays an important role, frowned upon Faraday's ideas. They looked upon electricity as an immaterial fluid that flows through matter; Faraday took a different point of view. He thought of it as a vibration, which was transmitted from place to place by intermediate contiguous particles. (Later physicists revived Descartes' idea of the ether to carry the vibrations.)
By 1850, Faraday's thinking had produced a radically new view of space: instead of being 'nothingness', a mere void in which were located various material objects. Rather, he saw it as a medium capable of supporting electric and magnetic forces, through collections of what he called lines of force. The forces were not localized in the particles which were the source of them; rather, their manifestation, the force lines, were to be found throughout the space around them. This marked the birth of field theory, which today is a key concept in all of physics. The collection of all the force lines forms a field of force, a term coined by William Thomson (later Lord Kelvin), who advocated and extended Faraday's ideas; initially, Faraday felt that Thomson was the only scientist who really understood his field ideas.[14]
Thomson's ideas were later taken up and extended and refined by James Clerk Maxwell, who maintained that the basic ideas for his mathematical theory of electromagnetic fields came from Faraday, and that his contribution was to turn them into a concise and elegant mathematical form.[10] Maxwell's characterization of his contributions, while admittedly first-hand, may be overly modest. 'Maxwell's equations', as they are now known, are today still the accepted form of the theory of electromagnetism. Among many other important results, they show that visible light consists of electromagnetic waves, as are radio waves, microwaves, infrared and gamma radiation, which are collectively known as electromagnetic radiation.
Last major discovery
Faraday was strong physically, but suffered occasionally from headaches, memory lapses, and bouts of depression. These symptoms increased in severity and frequency until in 1840, at the age of forty-nine, Faraday had a major breakdown, the exact nature of which is not certain. For four years he was hardly able to work, and his health never fully recovered.
However, by 1845 he was well enough to resume work, and he started his research activities again. Because he believed strongly in the unity of forces, he again investigated the effect of magnetic fields on light, an area he had previously investigated starting in 1822, but without success; in September, 1845, he made another major discovery. Acting on a suggestion by William Thomson, whose mathematical work on the Faraday's field ideas had produced a prediction that a magnetic field should affect polarized light,[17] he discovered such a connection; the polarization plane of polarized light is rotated by a magnetic field. This ability of magnetism to affect light is now known as the Faraday effect.
In the same series of experiments, he also discovered diamagnetism. Although these results did not have such important direct practical applications as some of his earlier work, there were of considerable importance in the development of electromagnetic theory.
Later life
True to his Sandemanian principles, and his indifference to honours and fame, Faraday turned down the offer of a knighthood, and twice declined to become president of the Royal Society. In 1861, when he was seventy years old, he resigned from the Royal Institution, but he was asked to stay on in a nominal post, which he did until 1865. In 1862, he and Sarah moved out from Albemarle Street into a house at Hampton Court provided a few years earlier by Queen Victoria, at the suggestion of her husband Prince Albert. He died there on August 25, 1867. In a characteristic display of his lifelong modesty, he had turned down an offer to be buried in Westminster Abbey, as he preferred a simpler funeral and grave (although he does have a memorial plaque there); he is buried in Highgate Cemetery.
Faraday is memorialized in a number of ways: in addition to the farad, an electrical unit named after him, statues of him stand at the Royal Institution, and outside the Institution of Electrical Engineers in London; a number of university buildings also bear his name. In perhaps his most notable honour, his image appeared on the British 20 pound banknote (see image at right) for some years—an honour given to only a very few scientists.
His successor in giving popular lectures on science at the Royal Institute, John Tyndall, who in 1853 became a professor at the Royal Institution, said of him:
Taking him for all and all, I think it will be conceded that Michael Faraday was the greatest experimental philosopher the world has ever seen; and I will add the opinion, that the progress of future research will tend, not to dim or to diminish, but to enhance and glorify the labours of this mighty investigator.
Time has fully confirmed the accuracy of Tyndall's estimation.
Faraday's science
This section contains some more technical detail on his most important results in physics and chemistry.
Electromagnetic rotation
When Faraday heard of Oersted's 1820 discovery that a steady electric current in a wire generates a cylindrical magnetic field (with the current-carrying wire as the axis of the cylinder), it occurred to him that a magnetic pole would be pushed around a circle by such a field. Hence, it would rotate forever, or at least as long as the current is flowing. He also reasoned that if a current in a wire can move a magnet, a magnet should be able to move a current-carrying wire. In 1821 he designed the apparatus shown in the figure on the right: it includes two distinct mechanisms, one for each of the two basic concepts he was working on. In one, electricity propels a moveable magnet, and in the other, a fixed magnet causes a mobile wire to move when electricity flows through it.[18]
In the vessel on the left, a strong bar magnet floats on end in a mercury bath, held in place only by a thread at its bottom. (Recall that mercury is a heavy, liquid, and metallic element that is a very good conductor of electricity.) A fixed copper wire dips into the mercury at the top of the bath; at the bottom of the vessel, another wire also projects into the mercury.
In the vessel on the right, another bar magnet is fixed in an upright position in the middle of another bath of mercury. A conducting (copper) socket extends into the bottom of the bath, and a copper wire which hangs from a flexible joint above the mercury bath dips into the top of the bath; the joint allows the top wire to pivot relatively freely around the joint.
When a direct current is switched on in either vessel (running in through one wire, through the mercury bath, and out through the other wire), it produces motion of the mechanism in that bath. The wire in the one on the right rotates around the magnet so fast that—as described by Faraday—the eye can scarcely follow the motion; the magnet on the left rotates around the fixed wire.
Note that Faraday's setup is such that only one pole of each of the two poles of the magnets is employed. If either of the magnets is turned around (i.e., the North and South poles are interchanged), the rotation which is observed in that vessel changes direction. The same happens if the direction of the current is reversed. Faraday, who coined the term electromagnetic rotation for this effect, had in fact invented a primitive precursor to the electric motor.
Electromagnetic induction
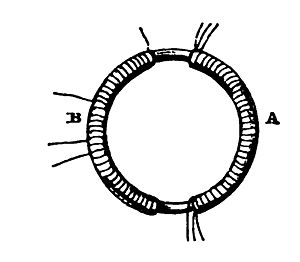
In a series of experiments performed in August, 1831, Faraday, knowing that electricity can create magnetism, investigated whether the converse effect is also true; in other words, whether magnetism has an effect on an electric current.
For this, he wound two coils of insulated wire around either side of a soft iron ring of six inches external diameter; the ring itself was 7/8 inch thick. (See the illustration on the left; the original apparatus for this, and other of his induction experiments, is preserved to this day in the Royal Institution.) The coils were not connected to each other. The coil A on the right-hand side could be connected to a battery; a copper wire attached to the coil B was led over a magnetic needle a few feet away from the ring. At the moment the battery was connected to coil A, and the current began to flow, the needle oscillated, and after a while settled into its original position. When the battery was disconnected from A, a disturbance of the needle was again observed—a result which surprised Faraday, who had not expected to see a pulse from both stopping as well as starting the flow of electricity.
When Faraday performed this experiment, it was already known that the part of the iron ring covered by coil A becomes a magnet—an electromagnet—when current runs through the coil. (The positioning of the test magnetic needle a few feet away was necessary to ensure that the electromagnetism of the powered coil A did not affect the magnetic needle.) The magnetic North and South poles of this electromagnet are at the beginning and the end of coil A—which end is North depends on the direction of the current in the coil A. The magnetic field produced by the electromagnet is effectively channeled from one end of it around to the other end through the iron of the ring, which is more hospitable to magnetic fields than air, a property known as magnetic permeability, thereby turning the entire ring into a magnet.
Since the iron ring passes through coil B, then as long as the current is flowing through coil A, B is wound around a magnet as well. Faraday had discovered that changes in the strength of the magnetic field of the electromagnet (which occur when switching it on and off) produces a current in the wire connected to coil B (which, remember, is not in electric contact with A). The proof that a current was running through B was the movement of the magnetic needle; it was known from Oersted's work that a current flowing in a wire will move a nearby magnetic needle.
Within a few weeks he further investigated this effect by setting up a cylindrical coil of about 4 centimeters diameter and about 16 centimeters long, made with 70 meters of wire. He then rapidly moved a permanent cylindrical bar magnet of about 22 centimeters length up and down inside the coil. He then was able to observe an electric current produced in the coil, 'induced' by the moving magnet. The direction of the current produced in the coil when the magnet was moved into the coil was the reverse of that produced when it was then pulled out. This important experiment proved that moving a wire through a magnetic field produced a current in the wire (because it was equivalent to holding the magnet still, and moving the coil instead).
He later succeeding in generating a current by rotating a copper disk between the poles of a large horseshoe magnet; the disk had one wire fixed to its center, and another made sliding contact along the edge of the disk. This was the first generator, and all generators today are its descendants. It also pointed the way toward the realization of an electric motor, since reversing the operation (by feeding an electric current into the disk) would make it rotate.
Faraday called this effect electromagnetic induction, and he was fascinated by the symmetry revealed by the effect that he now had discovered. Previously, it had been known that a moving electric charge (i.e. a current) produced a magnetic field; now it had been shown that a moving magnet produced an electric field (it is this field which causes the current to flow). Magnetic induction is of extreme importance in modern industrial society, because it is the principle behind electric generators and transformers.
Electrochemistry
The basic concept behind electrochemistry is quite simple: it consists of a vessel containing an electrolyte, which is a solution of charged particles (ions). A direct electric current is run through the electrolyte, introduced via electrodes which are dipped into it; the ensuing decomposition of chemicals in the solution is known as electrolysis. Depending on the electrolyte's chemical composition, and the makeup of the electrodes, a large range of useful chemical reactions result at the electrodes.
Since the solution is electrically neutral overall, the total charge carried by the positive particles (cations) is the same (in absolute value) as the total charge carried by the negative particles (anions). The current runs from the negatively charged electrode (the cathode) to the positively charged electrode (the anode). Inside the vessel, cations move to the cathode, pick up electrons—which carry negative charge—so that the cations are neutralized, and are deposited on the cathode. If the neutral product is gaseous, it escapes from the cathode in the form of gas. At the anode, the opposite happens: anions lose their excess electrons, and are deposited on the anode. Outside the vessel, the electrons run from the anode to the cathode.
Faraday's first law of electrochemistry states that the amount of substance deposited on the electrodes is proportional to the total amount of current that has passed through the electrodes. In the case of a steady current, this is equal to the time that the current has been running, multiplied by the amperage (flow rate) of the current. A constant proportional to this amount is named after him—Faraday's constant.
Faraday's second law of electrochemistry is a recognition of the fact that anions and cations may carry more than one elementary charges (although their number is always a small integer). Therefore, it takes a corresponding number of electrons to neutralize the cation, while for the anion to become neutral, it must lose a like number of electrons. These differently charged ions will all produce the deposition of only one atom from the requisite number of electrons, so that twice as much electricity is needed to generate an atom from a doubly charged ion as from a singly charged ion.
Faraday was the first to see these relationships clearly; moreover, all the terminology in the field (electrolysis, electrolyte, electrode, anode, cathode, ion, anion, and cation) was created by him.
Dielectrics and Faraday's cage
In his work on electrolysis, Faraday had noticed that many liquids which conduct an electric current become non-conducting when frozen. For instance, slightly acidic water is a good conductor, but when turned into ice is an insulator. Moreover, two plate electrodes with opposite electrical charge on them attract each other, even if a non-conducting substance—called by Faraday a dielectric—is in between the plates.
For Faraday this meant that the electrostatic attraction which produced that attractive force is not an action at a distance—as was generally assumed at the time—but a force conveyed by contiguous particles. In the second half of the 1830s he started a research program intended to prove these ideas about lines of force which are carried by intermediate particles (a concept later becoming important as ether), trying to disprove instantaneous action at a distance.
To prove his point about the nature of electrostatic forces, Faraday constructed capacitors of different shapes and sizes, and experimented with all kinds of dielectrics. To honor his work in this area, the SI unit of capacitance (the amount of charge that can be stored in a capacitor) is called a farad.
In the course of this research program, in November 1837, Faraday had a large wooden cube built, big enough to hold a person and some scientific apparatus, and had the sides completely covered with a network of conducting wires. He gives the following description of it:
I went into the cube and lived in it, and using lighted candles, electrometers, and all other tests of electrical states, I could not find the least influence upon them, or indication of anything particular given by them, though all the time the outside of the cube was powerfully charged, and large sparks and brushes were darting off from every part of its outer surface. [20]
The results of his experiments in the cube enabled him show that electricity was in fact a force rather than an imponderable fluid, as was argued by some physicists at that time. We would now call the conducting cube he constructed a Faraday cage; they are notorious among people trying to use cellular phones in buildings, and are a life-saver for car occupants in thunderstorms.
Further reading
- Cantor, Geoffrey N. Michael Faraday: Sandemanian and Scientist Macmillan (1991) - Explores the potential connections between Faraday's religious beliefs and his science.
- Cantor, G. N., David Gooding, and Frank A. J. L. James Michael Faraday Humanity Books, New York (1996) - A slim paperback, it still provides a good overview of Faraday as a person (including his religious beliefs), his scientific career and discoveries, and his influence; it also contains an interesting, brief, historiographical note.
- Hamilton, James A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution Random House, New York, (2004) ISBN 1-4000-6016-8 - Contains extensive quotations from original documents, and is well footnoted, but focuses more on Faraday the person than the details of his scientific work.
- Hirshfeld, Alan W. The Electric Life of Michael Faraday Walker (2006) - A modestly-sized volume that is more weighted toward the actual science than the Hamilton volume; includes an interesting chapter on the relationship between Faraday and Maxwell.
- James, Frank A. J. L. "Faraday, Michael (1791–1867)", Oxford Dictionary of National Biography, Sept 2004; online edn, Jan 2008
- Williams, L. Pearce Michael Faraday, A Biography Basic Books, New York (1965) - The first modern scholarly biography, this lengthy work covers his scientific career in considerable details, and contains extensive quotations from Faraday's original writings; each chapter ends with extensive source notes.
Notes
- ↑ Simmons, John G. The Scientific 100: A Ranking of the Most Influential Scientists, Past and Present | Complete chapter on Michael Faraday, pages 59-63. Google Books preview.
- ↑ [1] Press Release, University of Bath, 25 October 2006
- ↑ As a result he has been likened to Moses, in that he brought the scientific fields he worked in to a place he himself could not enter: an age when advanced mathematics became the language of science. See Simmons, John G. The Scientific 100: A Ranking of the Most Influential Scientists, Past and Present, pp. 59-60.
- ↑ Simmons, John G. The Scientific 100: A Ranking of the Most Influential Scientists, Past and Present, pg. 62.
- ↑ Some works give the name as Ribeau, but this seems to be incorrect.
- ↑ Hamilton, James A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution Random House, New York, (2004), pp. 10-12.
- ↑ Hamilton, James A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution, pg. 12.
- ↑ Faraday Heritage, Royal Institution
- ↑ J.H. Gladstone, Michael Faraday, (3rd ed, 1874). online edition
- ↑ 10.0 10.1 Faraday biography, Institute of Chemistry at the Hebrew University of Jerusalem.
- ↑ According to Discovery as Invention: Michael Faraday, this was theorized before by others, but he was the first to actually demonstrate it.
- ↑ 12.0 12.1 Frank A. J. L. James, "Faraday, Michael (1791–1867)", Oxford Dictionary of National Biography, Sept 2004; online edn, Jan 2008
- ↑ Hamilton, James A Life of Discovery: Michael Faraday, Giant of the Scientific Revolution, pp. 186-188.
- ↑ 14.0 14.1 Farndon, John et al. The Great Scientists: From Euclid to Stephen Hawking, Metro Books, New York (2007) pg. 82
- ↑ M. Faraday, The Chemical History Of A Candle (1908) online edition
- ↑ M. Faraday, On the Various Forces in Nature and their relations to each other: a course of lectures delivered before a juvenile audience at the Royal Institution, (six lectures)online
- ↑ Faraday biography, School of Mathematical and Computational Sciences at the University of St Andrews
- ↑ A modification of Plate IV in: M. Faraday, Experimental researches in electricity, vol II, Richard and John Edward Taylor, London (1844). On line
- ↑ Bence Jones, The Life and Letters of Faraday vol. II, online
- ↑ M. Faraday, Experimental researches in electricity, vol I, 2nd edition, Richard and John Edward Taylor, London (1849). p. 366 On line