Cefaclor: Difference between revisions
imported>David E. Volk m (stub) |
mNo edit summary |
||
| (10 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
'''Cefaclor''', also spelled as '''cephaclor''', is | {{Chem infobox | ||
|align=right | |||
|image=[[Image:Cefaclor.jpg|center|thumb|250px|{{#ifexist:Template:Cefaclor.jpg/credit|{{Cefaclor.jpg/credit}}<br/>|}}]] | |||
|width=250px | |||
|molname=cefaclor (cephaclor) | |||
|synonyms= cephaclor | |||
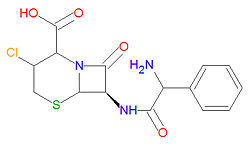
|molformula= C<sub>15</sub>H<sub>14</sub>ClN<sub>3</sub>O<sub>4</sub>S | |||
|molmass= 367.8074 | |||
|uses=antibiotic drug | |||
|properties= beta-lactam | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=53994-73-3 | |||
}} | |||
'''Cefaclor''', also spelled as '''cephaclor''', is a semisynthetic broad-spectrum [[antibiotic]] drug used to treat a variety of bacterial infections. It is a second generation [[cephalosporin]] antibiotic with similar activities. It is similar in structure to [[cephalexin]] and is also similar to penicillin-like drugs because it contains a [[beta-lactam]] moiety which binds to and interferes with bacterial cell wall synthesis. It can be used to treat a wide variety of both [[Gram-positive]] and [[Gram-negative]] aerobic bacteria. | |||
== Mechanism of action == | |||
Because cefaclor is a beta-lactam-based antibiotic, it binds to specific [[penicillin-binding protein]]s located inside the bacterial cell wall and inhibits the final stage of bacterial cell wall synthesis. As a consequence of the defective cell walls, the bacteria cells are autolysed by [[autolysin]]s, autolytic enzymes. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins. | |||
== Susceptible microbes == | |||
Among the Gram-positive bacteria, cefaclor has shown activity against [[Staphylococci]], including coagulase-(±) and penicillinase (+) strains, [[Streptococcus pneumoniae]], and [[Streptococcus pyogenes]]. Among the Gram-negative aerobes, cefaclor is active against [[Escherichia coli]], [[Haemophilus influenzae]], including ß-lactamase-producing ampicillin-resistant strains), [[Klebsiella sp]], and [[Proteus mirabilis]]. | |||
== Chemistry == | |||
The IUPAC chemical name for cefaclor is (6R,7R)-7-[(2-amino-2-phenylacetyl)amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, and it has chemical formula C<sub>15</sub>H<sub>14</sub>ClN<sub>3</sub>O<sub>4</sub>S giving it a molecular mass of 367.8074 g/mol. | |||
== Synonyms and brand names == | |||
{{col-begin}} | |||
{{col-break|width=25%}} | |||
''Synonyms'' | |||
* CCL | |||
* Cefaclor anhydrous | |||
* Cefaclorum (Latin) | |||
* Cephaclor | |||
{{col-break|width=25%}} | |||
''Brand names'' | |||
* Alenfral | |||
* Alfacet | |||
* Alfatil | |||
* Ceclor | |||
* Ceclor CD | |||
* Distaclor | |||
* Kefral | |||
* Panacef | |||
* Panoral | |||
* Raniclor | |||
{{col-end}} | |||
== External links == | |||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:01, 25 July 2024
|
| |||||||
| cefaclor (cephaclor) | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Cefaclor, also spelled as cephaclor, is a semisynthetic broad-spectrum antibiotic drug used to treat a variety of bacterial infections. It is a second generation cephalosporin antibiotic with similar activities. It is similar in structure to cephalexin and is also similar to penicillin-like drugs because it contains a beta-lactam moiety which binds to and interferes with bacterial cell wall synthesis. It can be used to treat a wide variety of both Gram-positive and Gram-negative aerobic bacteria.
Mechanism of action
Because cefaclor is a beta-lactam-based antibiotic, it binds to specific penicillin-binding proteins located inside the bacterial cell wall and inhibits the final stage of bacterial cell wall synthesis. As a consequence of the defective cell walls, the bacteria cells are autolysed by autolysins, autolytic enzymes. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins.
Susceptible microbes
Among the Gram-positive bacteria, cefaclor has shown activity against Staphylococci, including coagulase-(±) and penicillinase (+) strains, Streptococcus pneumoniae, and Streptococcus pyogenes. Among the Gram-negative aerobes, cefaclor is active against Escherichia coli, Haemophilus influenzae, including ß-lactamase-producing ampicillin-resistant strains), Klebsiella sp, and Proteus mirabilis.
Chemistry
The IUPAC chemical name for cefaclor is (6R,7R)-7-[(2-amino-2-phenylacetyl)amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, and it has chemical formula C15H14ClN3O4S giving it a molecular mass of 367.8074 g/mol.
Synonyms and brand names
|
Synonyms
|
Brand names
|
External links
The most up-to-date information about Cefaclor and other drugs can be found at the following sites.
- Cefaclor - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Cefaclor - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Cefaclor - Detailed information from DrugBank.