Adrenergic beta-agonist: Difference between revisions
imported>Robert Badgett |
mNo edit summary |
||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
'''Adrenergic beta-agonists''' ('''beta-agonists''') are "drugs that selectively bind to and activate beta-[[adrenergic receptor]]s."<ref>{{MeSH}}</ref> | '''Adrenergic beta-agonists''' ('''beta-agonists''') are "drugs that selectively bind to and activate beta-[[adrenergic receptor]]s."<ref>{{MeSH}}</ref> | ||
They are divided in several ways: short-acting (SABA) and [[Long acting adrenergic beta-agonists]] (LABA). | |||
==Medical uses== | ==Medical uses== | ||
===Asthma=== | ===Asthma=== | ||
:''See [[Asthma]]'' | :''See [[Asthma]]'' | ||
[[Image:SMART study primary outcomes, 2006.jpg|right|thumb|350px|{{#ifexist:Template:SMART study primary outcomes, 2006.jpg/credit|{{SMART study primary outcomes, 2006.jpg/credit}}<br/>|}}Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the ''primary outcome'' of the SMART study<ref name="pmid16424409">{{cite journal |author=Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM |title=The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol |journal=Chest |volume=129 | [[Image:SMART study primary outcomes, 2006.jpg|right|thumb|350px|{{#ifexist:Template:SMART study primary outcomes, 2006.jpg/credit|{{SMART study primary outcomes, 2006.jpg/credit}}<br/>|}}Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the ''primary outcome'' of the SMART study<ref name="pmid16424409">{{cite journal |author=Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM |title=The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol |journal=Chest |volume=129 |pages=15–26 |year=2006 |pmid=16424409 |doi=10.1378/chest.129.1.15 |url=http://www.chestjournal.org/cgi/pmidlookup?view=long&pmid=16424409 |issn=}}</ref> (serious adverse event - life threatening adverse events), controlling for race and use of concommitent steroids.]] | ||
[[Image:SMART study secondary outcomes, 2006.jpg|right|thumb|350px|{{#ifexist:Template:SMART study primary outcomes, 2006.jpg/credit|{{SMART study primary outcomes, 2006.jpg/credit}}<br/>|}}Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the ''secondary outcome'' of the SMART study<ref name="pmid16424409" | [[Image:SMART study secondary outcomes, 2006.jpg|right|thumb|350px|{{#ifexist:Template:SMART study primary outcomes, 2006.jpg/credit|{{SMART study primary outcomes, 2006.jpg/credit}}<br/>|}}Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the ''secondary outcome'' of the SMART study<ref name="pmid16424409" /> (asthma-related mortality), controlling for race and use of concommitent steroids.]] | ||
Among patients with asthma, LABAs | Among patients with asthma, LABAs tend to increase serious adverse effects and mortality. This harm is reduced, but not definitely eliminated, when LABAs are combined with [[corticosteroid]]s.<ref name="pmid18646149">{{cite journal |author=Cates CJ, Cates MJ |title=Regular treatment with salmeterol for chronic asthma: serious adverse events |journal=Cochrane Database Syst Rev |volume= |issue=3 |pages=CD006363 |year=2008 |pmid=18646149 |doi=10.1002/14651858.CD006363.pub2 |url=http://dx.doi.org/10.1002/14651858.CD006363.pub2 |issn=}}</ref> | ||
===Chronic obstructive pulmonary disease=== | ===Chronic obstructive pulmonary disease=== | ||
| Line 16: | Line 16: | ||
==Drug toxicity== | ==Drug toxicity== | ||
Long acting adrenergic beta-agonists (LABA) associated with may adverse outcomes among patients with ''asthma'' when used ''without'' [[corticosteroid]]s and maybe among African American patients.<ref name="pmid16754916">{{cite journal |author=Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE |title=Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths |journal=Ann. Intern. Med. |volume=144 |issue=12 |pages=904-12 |year=2006 |pmid=16754916 |doi= |url=http://www.annals.org/cgi/pmidlookup?view=reprint&pmid=16754916 |issn=}}</ref> They might be safe in asthma as long as [[corticosteroid]]s are used. According to a [[meta-analysis|meta-analyses]] by the [[Cochrane Collaboration]], when used ''with'' [[corticosteroid]]s the relative risk for asthma-related death is increased at 1.34 although this increase was not statistically significant with a [[confidence interval]] of 0.30 to 5.97.<ref name="pmid17253458">{{cite journal |author=Walters EH, Gibson PG, Lasserson TJ, Walters JA |title=Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid |journal=Cochrane Database Syst Rev |volume= |issue=1 |pages=CD001385 |year=2007 |pmid=17253458 |doi=10.1002/14651858.CD001385.pub2 |url=http://dx.doi.org/10.1002/14651858.CD001385.pub2 |issn=}}</ref><ref name="pmid18646149">{{cite journal |author=Cates CJ, Cates MJ |title=Regular treatment with salmeterol for chronic asthma: serious adverse events |journal=Cochrane Database Syst Rev |volume= |issue=3 |pages=CD006363 |year=2008 |pmid=18646149 |doi=10.1002/14651858.CD006363.pub2 |url=http://dx.doi.org/10.1002/14651858.CD006363.pub2 |issn=}}</ref> There are two major [[randomized controlled trial]]s of LABAs. The SNS study compared salmeterol to salbutamol<ref name="pmid8098238">{{cite journal |author=Castle W, Fuller R, Hall J, Palmer J |title=Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment |journal=BMJ |volume=306 |issue=6884 |pages=1034–7 |year=1993 |month=April |pmid=8098238 |pmc=1676982 |doi= |url= |issn=}}</ref> while the SMART study compared salmeterol to placebo.<ref name="pmid16424409" | Long acting adrenergic beta-agonists (LABA) associated with may adverse outcomes among patients with ''asthma'' when used ''without'' [[corticosteroid]]s and maybe among African American patients.<ref name="pmid16754916">{{cite journal |author=Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE |title=Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths |journal=Ann. Intern. Med. |volume=144 |issue=12 |pages=904-12 |year=2006 |pmid=16754916 |doi= |url=http://www.annals.org/cgi/pmidlookup?view=reprint&pmid=16754916 |issn=}}</ref> They might or might not<ref name="pmid20176343">{{cite journal| author=Salpeter SR, Wall AJ, Buckley NS| title=Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. | journal=Am J Med | year= 2010 | volume= 123 | issue= 4 | pages= 322-8.e2 | pmid=20176343 | ||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=20176343 | doi=10.1016/j.amjmed.2009.07.035 }} </ref> be safe in asthma as long as [[corticosteroid]]s are used. According to a [[meta-analysis|meta-analyses]] by the [[Cochrane Collaboration]], when used ''with'' [[corticosteroid]]s the relative risk for asthma-related death is increased at 1.34 although this increase was not statistically significant with a [[confidence interval]] of 0.30 to 5.97.<ref name="pmid17253458">{{cite journal |author=Walters EH, Gibson PG, Lasserson TJ, Walters JA |title=Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid |journal=Cochrane Database Syst Rev |volume= |issue=1 |pages=CD001385 |year=2007 |pmid=17253458 |doi=10.1002/14651858.CD001385.pub2 |url=http://dx.doi.org/10.1002/14651858.CD001385.pub2 |issn=}}</ref><ref name="pmid18646149">{{cite journal |author=Cates CJ, Cates MJ |title=Regular treatment with salmeterol for chronic asthma: serious adverse events |journal=Cochrane Database Syst Rev |volume= |issue=3 |pages=CD006363 |year=2008 |pmid=18646149 |doi=10.1002/14651858.CD006363.pub2 |url=http://dx.doi.org/10.1002/14651858.CD006363.pub2 |issn=}}</ref> There are two major [[randomized controlled trial]]s of LABAs. The SNS study compared salmeterol to salbutamol<ref name="pmid8098238">{{cite journal |author=Castle W, Fuller R, Hall J, Palmer J |title=Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment |journal=BMJ |volume=306 |issue=6884 |pages=1034–7 |year=1993 |month=April |pmid=8098238 |pmc=1676982 |doi= |url= |issn=}}</ref> while the SMART study compared salmeterol to placebo.<ref name="pmid16424409" /> | |||
The varying response to beta adrenergic agonists in African Americans may be due to a polymorphism in African-American patients of the G protein–coupled [[cell surface receptor]] kinase (GRK5)([http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=600870 OMIM]) that confers a natural "genetic beta-blockade".<ref name="pmid18622265">{{cite journal |author=Wang WC, Mihlbachler KA, Bleecker ER, Weiss ST, Liggett SB |title=A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors |journal=Pharmacogenet. Genomics |volume=18 |issue=8 |pages=729–32 |year=2008 |month=August |pmid=18622265 |doi=10.1097/FPC.0b013e32830967e9 |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?an=01213011-200808000-00009 |issn=}}</ref> | The varying response to beta adrenergic agonists in African Americans may be due to a polymorphism in African-American patients of the G protein–coupled [[cell surface receptor]] kinase (GRK5)([http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=600870 OMIM]) that confers a natural "genetic beta-blockade".<ref name="pmid18622265">{{cite journal |author=Wang WC, Mihlbachler KA, Bleecker ER, Weiss ST, Liggett SB |title=A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors |journal=Pharmacogenet. Genomics |volume=18 |issue=8 |pages=729–32 |year=2008 |month=August |pmid=18622265 |doi=10.1097/FPC.0b013e32830967e9 |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?an=01213011-200808000-00009 |issn=}}</ref> | ||
==Ozone depletion== | |||
In order to comply with ozone protection, these medicines must not use chlorofluorocarbons (CFC) for a chlorofluorocarbon by January 1, 2009.<ref name="urlInformation on the Elimination of Chlorofluorocarbon-containing (CFC) Albuterol MDIs and Other Ozone-Depleting Drug Products">{{cite web |url=http://www.fda.gov/cder/mdi/albuterol.htm |title=Information on the Elimination of Chlorofluorocarbon-containing (CFC) Albuterol MDIs and Other Ozone-Depleting Drug Products |author=Anonymous |authorlink= |coauthors= |date=2008 |format= |work= |publisher=Food and Drug Administration |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref><ref name="New York Times">{{cite web |url=http://www.nytimes.com/2008/05/13/health/13asth.html |title=Rough Transition to a New Asthma Inhaler |author=Tarkan L |authorlink= |coauthors= |date=May 13, 2008 |format= |work= |publisher=New York Times |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2008-05-13}}</ref> Inhalers using hydrofluoroalkane (HFA) propellants are CFC-free. The HFA based inhalers are brand name and more expensive. They are Ventolin by GlaxoSmithKline, ProAir by Ivax, Proventil by Schering-Plough and Xopenex by Sepracor. The first three use [[albuterol]] whereas Xopenex uses [[levalbuterol]]. Financial assistance is available for patients available through the Partnership for Prescription Assistance (1-888-477-2669).<ref name="New York Times"/> As of November, 2008, the prices costs in American dollars were Ventolin 36.18, ProAir 33.92, Proventil 41.34 and Xopenex 52.50.<ref name="Medical Letter2008">{{cite web |url=http://www.medicalletter.org/restricted/articles/w1298c.pdf |title=New Propellants for Albuterol Metered-Dose | |||
Inhalers|date=November, 2008|volume=50 |issue=1298 |pages=85|format=pdf |publisher=Medical Letter|work= |accessdate=}}</ref> | |||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:00, 6 July 2024
Adrenergic beta-agonists (beta-agonists) are "drugs that selectively bind to and activate beta-adrenergic receptors."[1]
They are divided in several ways: short-acting (SABA) and Long acting adrenergic beta-agonists (LABA).
Medical uses
Asthma
- See Asthma
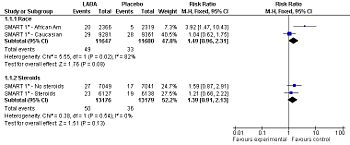
Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the primary outcome of the SMART study[2] (serious adverse event - life threatening adverse events), controlling for race and use of concommitent steroids.
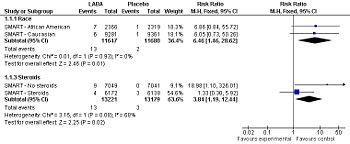
Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the secondary outcome of the SMART study[2] (asthma-related mortality), controlling for race and use of concommitent steroids.
Among patients with asthma, LABAs tend to increase serious adverse effects and mortality. This harm is reduced, but not definitely eliminated, when LABAs are combined with corticosteroids.[3]
Chronic obstructive pulmonary disease
Among patients with COPD, reduction in mortality may not occur unless LABAs are combined with corticosteroids.[4]
Drug toxicity
Long acting adrenergic beta-agonists (LABA) associated with may adverse outcomes among patients with asthma when used without corticosteroids and maybe among African American patients.[5] They might or might not[6] be safe in asthma as long as corticosteroids are used. According to a meta-analyses by the Cochrane Collaboration, when used with corticosteroids the relative risk for asthma-related death is increased at 1.34 although this increase was not statistically significant with a confidence interval of 0.30 to 5.97.[7][3] There are two major randomized controlled trials of LABAs. The SNS study compared salmeterol to salbutamol[8] while the SMART study compared salmeterol to placebo.[2]
The varying response to beta adrenergic agonists in African Americans may be due to a polymorphism in African-American patients of the G protein–coupled cell surface receptor kinase (GRK5)(OMIM) that confers a natural "genetic beta-blockade".[9]
Ozone depletion
In order to comply with ozone protection, these medicines must not use chlorofluorocarbons (CFC) for a chlorofluorocarbon by January 1, 2009.[10][11] Inhalers using hydrofluoroalkane (HFA) propellants are CFC-free. The HFA based inhalers are brand name and more expensive. They are Ventolin by GlaxoSmithKline, ProAir by Ivax, Proventil by Schering-Plough and Xopenex by Sepracor. The first three use albuterol whereas Xopenex uses levalbuterol. Financial assistance is available for patients available through the Partnership for Prescription Assistance (1-888-477-2669).[11] As of November, 2008, the prices costs in American dollars were Ventolin 36.18, ProAir 33.92, Proventil 41.34 and Xopenex 52.50.[12]
References
- ↑ Anonymous (2024), Adrenergic beta-agonist (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ 2.0 2.1 2.2 Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM (2006). "The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol". Chest 129: 15–26. DOI:10.1378/chest.129.1.15. PMID 16424409. Research Blogging.
- ↑ 3.0 3.1 Cates CJ, Cates MJ (2008). "Regular treatment with salmeterol for chronic asthma: serious adverse events". Cochrane Database Syst Rev (3): CD006363. DOI:10.1002/14651858.CD006363.pub2. PMID 18646149. Research Blogging.
- ↑ Rodrigo GJ, Nannini LJ, Rodríguez-Roisin R (May 2008). "Safety of Long-Acting {beta}-Agonists in Stable COPD: A Systematic Review". Chest 133 (5): 1079–87. DOI:10.1378/chest.07-1167. PMID 18460518. Research Blogging.
- ↑ Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE (2006). "Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths". Ann. Intern. Med. 144 (12): 904-12. PMID 16754916. [e]
- ↑ Salpeter SR, Wall AJ, Buckley NS (2010). "Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events.". Am J Med 123 (4): 322-8.e2. DOI:10.1016/j.amjmed.2009.07.035. PMID 20176343. Research Blogging.
- ↑ Walters EH, Gibson PG, Lasserson TJ, Walters JA (2007). "Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid". Cochrane Database Syst Rev (1): CD001385. DOI:10.1002/14651858.CD001385.pub2. PMID 17253458. Research Blogging.
- ↑ Castle W, Fuller R, Hall J, Palmer J (April 1993). "Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment". BMJ 306 (6884): 1034–7. PMID 8098238. PMC 1676982. [e]
- ↑ Wang WC, Mihlbachler KA, Bleecker ER, Weiss ST, Liggett SB (August 2008). "A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors". Pharmacogenet. Genomics 18 (8): 729–32. DOI:10.1097/FPC.0b013e32830967e9. PMID 18622265. Research Blogging.
- ↑ Anonymous (2008). Information on the Elimination of Chlorofluorocarbon-containing (CFC) Albuterol MDIs and Other Ozone-Depleting Drug Products. Food and Drug Administration.
- ↑ 11.0 11.1 Tarkan L (May 13, 2008). Rough Transition to a New Asthma Inhaler. New York Times. Retrieved on 2008-05-13.
- ↑ [http://www.medicalletter.org/restricted/articles/w1298c.pdf New Propellants for Albuterol Metered-Dose Inhalers] (pdf) 85. Medical Letter (November, 2008).