N-hydroxysuccinimide: Difference between revisions
imported>David E. Volk (New page: {{subpages}} {{Image|NHS.png|right|150px|N-hydroxysuccinimide}} '''N-hydroxysuccinimide''' (NHS) is chemical used to form semi-stable, but reactive, esters from carboxylic acids. ...) |
mNo edit summary |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 2: | Line 2: | ||
{{Image|NHS.png|right|150px|N-hydroxysuccinimide}} | {{Image|NHS.png|right|150px|N-hydroxysuccinimide}} | ||
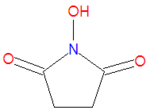
'''N-hydroxysuccinimide''' (NHS) is chemical used to form semi-stable, but reactive, [[ester]]s from [[carboxylic acid]]s. Such NHS esters can be stored for a relatively long time if kept cold and dry. NHS is typically used in conjunction with a dehydrating | '''N-hydroxysuccinimide''' (NHS) is chemical used to form semi-stable, but reactive, [[ester]]s from [[carboxylic acid]]s. Such NHS esters can be stored for a relatively long time if kept cold and dry. NHS is typically used in conjunction with a dehydrating reactant, often a [[carbodiimide]] such as [[EDAC]], [[DCC]] or [[DIC]]. A very similar compound, sulfo-NHS, which contains an SO<sub>3</sub><sup>-</sup> group on one of the two aliphatic carbons, is often used instead. | ||
== Carboxylate Activation with Carbodiimide and NHS == | |||
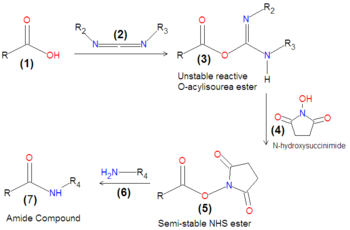
{{Image|Carbodiimide coupling via NHS ester.png|left|350px|'''Scheme 1''':Coupling of a carboxylic acid and a primary amine via O-acylisourea ester and NHS ester intermediates.}} | |||
As depicted in '''Scheme 1''', the carbodiimide-mediated coupling of a carboxylic acid ('''1''') with a primary amine first progresses through an unstable intermediate, an O-acylisourea ester ('''3''') compound formed by the reaction of the carbodiimide ('''2''') with the carboxylic acid ('''1'''). Because this intermediate is unstable, it is often the case that either NHS ([[N-hydroxysuccinimide]]) '''4''' or sulfo-NHS is added to the carboxylic acid together with the carbodiimide, in order to form an NHS-ester ('''5'''), which is a much more stable compound that remains reactive with amines. Upon the final addition of an amine ('''6'''), the final desired coupling product, an amide ('''7'''), is formed by displacement of the NHS.[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 22 September 2024
N-hydroxysuccinimide (NHS) is chemical used to form semi-stable, but reactive, esters from carboxylic acids. Such NHS esters can be stored for a relatively long time if kept cold and dry. NHS is typically used in conjunction with a dehydrating reactant, often a carbodiimide such as EDAC, DCC or DIC. A very similar compound, sulfo-NHS, which contains an SO3- group on one of the two aliphatic carbons, is often used instead.
Carboxylate Activation with Carbodiimide and NHS
As depicted in Scheme 1, the carbodiimide-mediated coupling of a carboxylic acid (1) with a primary amine first progresses through an unstable intermediate, an O-acylisourea ester (3) compound formed by the reaction of the carbodiimide (2) with the carboxylic acid (1). Because this intermediate is unstable, it is often the case that either NHS (N-hydroxysuccinimide) 4 or sulfo-NHS is added to the carboxylic acid together with the carbodiimide, in order to form an NHS-ester (5), which is a much more stable compound that remains reactive with amines. Upon the final addition of an amine (6), the final desired coupling product, an amide (7), is formed by displacement of the NHS.