Aromatic hydrocarbons: Difference between revisions
imported>Mary Ash (weaving the links) |
mNo edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC|right}} | {{TOC|right}} | ||
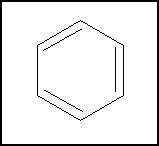
'''Aromatic hydrocarbons''' ( | '''Aromatic hydrocarbons''' (also called '''arenes''') are a class of cyclic [[hydrocarbons]] with complete conjugation (which often means alternating double and single bonds) in the molecular rings. They tend to be more stable than normal [[alkene]]s. Not all fully conjugated cyclic hydrocarbons are [[aromatic]], however- some act like normal alkenes, and one ([[cyclobutadiene]]) is anti-aromatic, and is even less stable than a normal alkene. The reasons for this are discussed below, under theoretical considerations. The most common aromatic hydrocarbon is [[benzene]], which was also the first to be discovered. Many larger aromatics consist of multiple [[benzene]] rings fused together. Benzene, alkyl benzenes (such as [[toluene]]), and fused-ring aromatics (such as [[anthracene]]) can all be isolated from coal tar. There are also [[aromatic compound]]s with [[chemical element]]s other than [[carbon]] and [[hydrogen]], so they are aromatic but not hydrocarbons. | ||
Simple aromatic hydrocarbons produced in bulk quantities are often [[petrochemical]]s which are separated (sometimes only partially) or produced from [[petroleum]] i.e. crude oil from the ground. Other sources include coal tar. Such petrochemical aromatic hydrocarbons or mixtures of them are used extensively in the chemical industry, as solvents, as [[gasoline]] additives or components to raise [[octane number]] without undesirable lead compounds, and in the [[monomer]]/[[polymer]] industry. | |||
==Important industrial application== | |||
In [[Petroleum refinery|petroleum refining]], aromatic hydrocarbon petrochemicals are commonly produced by a process called [[catalytic reforming]] or, to yield a more concentrated arene mixture, an aromatizing version of this process. BTX refers to a mixture of Benzene, Toluene, and three types or [[isomer]]s of [[Xylene]]s. Such arene mixtures often have [[Ethylbenzene]] also. Compounds from this sort of mixture can be added to gasoline to increase octane number. Benzene can be methylated (an -[[Hydrogen|H]] changed to [[Methyl group|-CH<sub>3</sub> group]]) once to give toluene or twice to give a xylene. Benzene can also react with [[ethylene]] to give [[ethylbenzene]], which in turn can give [[styrene]] and [[hydrogen]] ( H<sub>2</sub>). Styrene is commonly used as a monomer for polymerization to [[polystyrene]], sometimes with some [[divinylbenzene]] for cross-linking, to a styrene-[[1,3-Butadiene|butadiene]] copolymer, or ABS, an [[acrylonitrile]]-butadiene-styrene copolymer. | |||
The three isomers of xylene are ''ortho''-, ''meta''-, and ''para''-xylene. There can be isomerization units to interconvert xylenes in a mixture to an equilibrium mixture of the three isomers. There are separation methods to separate the xylenes. The ''para''-xylene is the most desired because it can be oxidized to [[terephthalic acid]] which is then polymerized to the very useful plastic [[polyethylene terephthalate]]. | |||
A cumene process has been used to convert [[cumene]] (isopropylbenzene) to [[phenol]] and [[acetone]]. | |||
==Reactivity== | ==Reactivity== | ||
{{Image|Benzene DEVolk.jpg|right|350px|<small>Benzene, the simplest aromatic</small>}} | {{Image|Benzene DEVolk.jpg|right|350px|<small>Benzene, the simplest aromatic hydrocarbon</small>}} | ||
While aromatic hydrocarbons appear to contain multiple double bonds, they undergo none of the reactions typical of | While aromatic hydrocarbons appear to contain multiple double bonds, they undergo none of the reactions typical of alkenes. [[Catalyst|Catalytic]] addition of [[hydrogen]] to aromatics requires either higher pressures of hydrogen or more powerful [[catalyst]]s than does addition to alkenes. [[Water]] will add to alkenes with [[acid]] [[catalysis]], but will not add to aromatic rings. Some larger aromatic rings will undergo reactions more typical of alkenes. One of those that will is the middle ring of anthracene. | ||
In general,[[electrophile|electrophiles]] replace a hydrogen atom of an aromatic ring instead of adding to the double bonds. The most common reactions include: | In general,[[electrophile|electrophiles]] replace a hydrogen atom of an aromatic ring instead of adding to the double bonds. The most common reactions include: | ||
;'''Nitration''': A mixture of [[nitric acid|nitric]] and [[sulfuric acid|sulfuric]] acids is used to replace a hydrogen atom with a [[nitro]] (NO<sub>2</sub>) group. This can be reduced to an [[amine]] if desired. | ;'''Nitration''': A mixture of [[nitric acid|nitric]] and [[sulfuric acid|sulfuric]] acids is used to replace a hydrogen atom with a [[nitro]] (-NO<sub>2</sub>) group. This can be reduced to an [[amine]] if desired. | ||
;'''Sulfonation''': Oleum, a mixture of very concentrated sulfuric acid and sulfur trioxide, can be used to replace a hydrogen with a SO<sub>3</sub>H group. These [[sulfonic acids]] are used to make [[sulfonamide|sulfa drugs]], and are also strong acids soluble in organic solvents (unlike some of the strong mineral acids) | ;'''Sulfonation''': Oleum, a mixture of very concentrated sulfuric acid and sulfur trioxide, can be used to replace a hydrogen with a -SO<sub>3</sub>H group. These [[sulfonic acids]] are used to make [[sulfonamide|sulfa drugs]], and are also strong acids soluble in organic solvents (unlike some of the strong mineral acids) | ||
;'''Friedel-Crafts acylation''': This process uses a carboxylic acid chloride and AlCl<sub>3</sub> to introduce an acyl group, which forms a benzyl [[ketone]]. This can be reduced to an alkylbenzene if desired. | ;'''Friedel-Crafts acylation''': This process uses a carboxylic acid chloride and AlCl<sub>3</sub> to introduce an acyl group, which forms a benzyl [[ketone]]. This can be reduced to an alkylbenzene if desired. | ||
;Friedel-Crafts alkylation:This process, which uses an alkyl halide and AlCl<sub>3</sub> catalyst to introduce an alkyl group, was discovered before the acylation reaction (see above). However, the alkylation is less reliable, so acylation is often used instead. | ;Friedel-Crafts alkylation:This process, which uses an alkyl halide and AlCl<sub>3</sub> catalyst to introduce an alkyl group, was discovered before the acylation reaction (see above). However, the alkylation is less reliable, so acylation is often used instead. | ||
| Line 16: | Line 25: | ||
==Theoretical Considerations== | ==Theoretical Considerations== | ||
<nowiki></nowiki> | |||
<nowiki></nowiki> | |||
<nowiki></nowiki> | |||
<nowiki></nowiki>[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 13 July 2024
Aromatic hydrocarbons (also called arenes) are a class of cyclic hydrocarbons with complete conjugation (which often means alternating double and single bonds) in the molecular rings. They tend to be more stable than normal alkenes. Not all fully conjugated cyclic hydrocarbons are aromatic, however- some act like normal alkenes, and one (cyclobutadiene) is anti-aromatic, and is even less stable than a normal alkene. The reasons for this are discussed below, under theoretical considerations. The most common aromatic hydrocarbon is benzene, which was also the first to be discovered. Many larger aromatics consist of multiple benzene rings fused together. Benzene, alkyl benzenes (such as toluene), and fused-ring aromatics (such as anthracene) can all be isolated from coal tar. There are also aromatic compounds with chemical elements other than carbon and hydrogen, so they are aromatic but not hydrocarbons.
Simple aromatic hydrocarbons produced in bulk quantities are often petrochemicals which are separated (sometimes only partially) or produced from petroleum i.e. crude oil from the ground. Other sources include coal tar. Such petrochemical aromatic hydrocarbons or mixtures of them are used extensively in the chemical industry, as solvents, as gasoline additives or components to raise octane number without undesirable lead compounds, and in the monomer/polymer industry.
Important industrial application
In petroleum refining, aromatic hydrocarbon petrochemicals are commonly produced by a process called catalytic reforming or, to yield a more concentrated arene mixture, an aromatizing version of this process. BTX refers to a mixture of Benzene, Toluene, and three types or isomers of Xylenes. Such arene mixtures often have Ethylbenzene also. Compounds from this sort of mixture can be added to gasoline to increase octane number. Benzene can be methylated (an -H changed to -CH3 group) once to give toluene or twice to give a xylene. Benzene can also react with ethylene to give ethylbenzene, which in turn can give styrene and hydrogen ( H2). Styrene is commonly used as a monomer for polymerization to polystyrene, sometimes with some divinylbenzene for cross-linking, to a styrene-butadiene copolymer, or ABS, an acrylonitrile-butadiene-styrene copolymer.
The three isomers of xylene are ortho-, meta-, and para-xylene. There can be isomerization units to interconvert xylenes in a mixture to an equilibrium mixture of the three isomers. There are separation methods to separate the xylenes. The para-xylene is the most desired because it can be oxidized to terephthalic acid which is then polymerized to the very useful plastic polyethylene terephthalate.
A cumene process has been used to convert cumene (isopropylbenzene) to phenol and acetone.
Reactivity
While aromatic hydrocarbons appear to contain multiple double bonds, they undergo none of the reactions typical of alkenes. Catalytic addition of hydrogen to aromatics requires either higher pressures of hydrogen or more powerful catalysts than does addition to alkenes. Water will add to alkenes with acid catalysis, but will not add to aromatic rings. Some larger aromatic rings will undergo reactions more typical of alkenes. One of those that will is the middle ring of anthracene.
In general,electrophiles replace a hydrogen atom of an aromatic ring instead of adding to the double bonds. The most common reactions include:
- Nitration
- A mixture of nitric and sulfuric acids is used to replace a hydrogen atom with a nitro (-NO2) group. This can be reduced to an amine if desired.
- Sulfonation
- Oleum, a mixture of very concentrated sulfuric acid and sulfur trioxide, can be used to replace a hydrogen with a -SO3H group. These sulfonic acids are used to make sulfa drugs, and are also strong acids soluble in organic solvents (unlike some of the strong mineral acids)
- Friedel-Crafts acylation
- This process uses a carboxylic acid chloride and AlCl3 to introduce an acyl group, which forms a benzyl ketone. This can be reduced to an alkylbenzene if desired.
- Friedel-Crafts alkylation
- This process, which uses an alkyl halide and AlCl3 catalyst to introduce an alkyl group, was discovered before the acylation reaction (see above). However, the alkylation is less reliable, so acylation is often used instead.
- Halogenation
- This uses Br2 or Cl2, along with AlCl3 or FeBr3 as a catalyst, to introduce a bromine or chlorine atom.
Theoretical Considerations