Carbon monoxide: Difference between revisions
John Leach (talk | contribs) m (Text replacement - "hydrogen sulfide" to "hydrogen sulphide") |
mNo edit summary |
||
| Line 112: | Line 112: | ||
<ref name=Eilert>S.J. Eilert (September 2005), "New packaging technologies for the 21st century", <i>Journal of Meat Science</i>, Vol. 71, Issue 1, pp. 122–127.</ref> | <ref name=Eilert>S.J. Eilert (September 2005), "New packaging technologies for the 21st century", <i>Journal of Meat Science</i>, Vol. 71, Issue 1, pp. 122–127.</ref> | ||
<ref name=FoodSafety>[http://www.foodsafetymagazine.com/article.asp?id=644&sub=sub1 Low-Oxygen Packaging With CO: A Study in Food Politics That Warrants Peer Review], Randall D. Huffman and Janet M. Riley, Food Safety Magazine, Dec 2006/Jan 2007.</ref> | <ref name=FoodSafety>[http://www.foodsafetymagazine.com/article.asp?id=644&sub=sub1 Low-Oxygen Packaging With CO: A Study in Food Politics That Warrants Peer Review], Randall D. Huffman and Janet M. Riley, Food Safety Magazine, Dec 2006/Jan 2007.</ref> | ||
}} | }}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:00, 25 July 2024
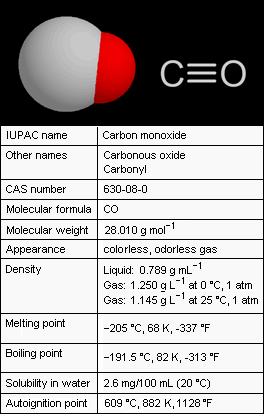
Carbon monoxide (CO), also referred to as carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. Exposure to high levels of carbon monoxide is extremely toxic to humans and animals. Conversely, small amounts of carbon monoxide are produced in normal animal metabolism and it is thought to have some normal biological functions.
Carbon monoxide consists of one carbon atom and one oxygen atom. It is the simplest member of the class of inorganic compounds known as oxocarbons which includes carbon dioxide (CO2), carbon suboxide (C3O2), mellitic anhydride (C12O9) and many others. When combined with a metal (i.e., an organometallic complex), the carbon monoxide is a ligand called carbonyl : for example, in nickel carbonyl with the formula Ni(CO)4.
Carbon monoxide is produced by the partial combustion of carbon-containing substances. It is produced when there is not enough oxygen to form carbon dioxide, such as when operating a stove or an internal combustion engine in an enclosed space.
In the presence of oxygen, carbon monoxide burns with a blue flame, producing carbon dioxide.[1] Coal gas, which was widely used before the 1960s for domestic lighting, cooking, and heating, had carbon monoxide as a significant constituent. Iron smelting and other current technological processes still produce byproduct carbon monoxide.[2]
Worldwide, the largest source of carbon monoxide is from the photochemical reactions in the troposphere that generate about 5 x 1012 kilograms per year.[3] Other natural sources of CO include volcanoes, forest fires, and other forms of combustion.
Toxicity
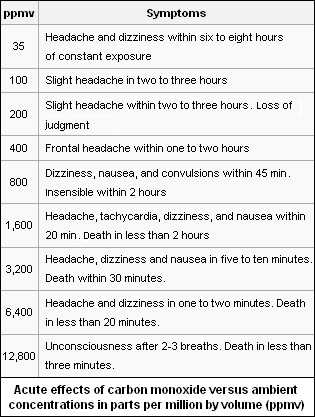
Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries. It combines with hemoglobin in the red blood cells of humans and animals to produce carboxy hemoglobin, which is ineffective for delivering oxygen to bodily tissues. Exposure to carbon monoxide levels as low as 667 parts per million by volume (ppmv) may cause up to 50 % of the body's hemoglobin to convert to carboxyhemoglobin[4] which may result in seizure, coma, and fatality.
The adjacent table lists the typical symptoms caused by exposure to various concentrations of carbon monoxide, expressed in parts per million by volume (ppmv).
On average, exposures to levels of 100 ppm or greater is dangerous to human health. In the United States of America, the long-term workplace exposure levels are limited by the Occupational Safety and Health Administration (OSHA) to less than 50 ppmv averaged over an 8-hour period;[5] in addition, employees are to be removed from any confined space if an upper limit (ceiling) of 100 ppmv is reached.
The most common symptoms of carbon monoxide poisoning may resemble other types of poisonings and infections, including symptoms such as headache, nausea, vomiting, dizziness, fatigue, and a feeling of weakness. Infants may be irritable and feed poorly.[6]
Neurological symptoms include confusion, disorientation, visual disturbance, fainting and seizures.
Exposures to carbon monoxide may cause significant damage to the heart and central nervous system, especially to a sub-cortical component of the brain, often with long-term effects.
Carbon monoxide may have severe adverse effects on the fetus of a pregnant woman.
Sources and occurrences of carbon monoxide
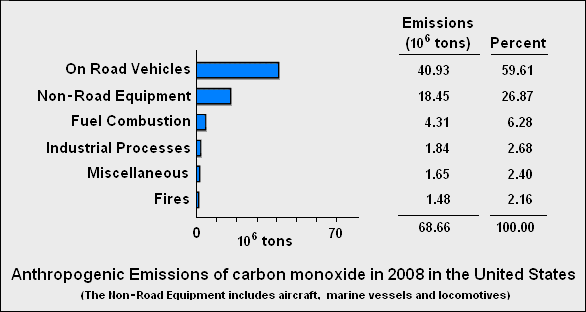
As mentioned above, the largest sources of carbon emissions are natural in origin. The anthropogenic (man-made) sources are quite small by comparison.
The graph below lists the anthropogenic sources of carbon monoxide in the United States during 2008:[7]
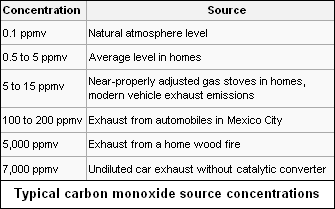
Typical source concentrations of carbon monoxide
Carbon monoxide occurs in various natural and artificial environments. Typical concentrations in parts per million by volume are as follows:[8][9][10]
Atmosphere
Carbon monoxide at low levels occurs in the atmosphere, primarily as a product of volcanic activity but also from natural and man-made fires such as slash and burn agriculture, burning of crop residues, and sugarcane fire-cleaning. Combustion of fossil fuels also results in carbon monoxide emissions to the atmosphere.
Through natural processes in the atmosphere, carbon monoxide is eventually converted to carbon dioxide. Carbon monoxide concentrations in the atmosphere are short-lived and geographically variable.
Urban areas
Carbon monoxide occurs as a major atmospheric pollutant in most urban areas, chiefly from the engine exhausts of vehicles, portable and back-up generators, lawn mowers, leaf blowers, etc. One of the most important cases of carbon monoxide buildup is in the presence of urban street canyons or other semi-enclosed volumes created by man-made structures. The incomplete combustion of various other fuels such as natural gas, fuel oils, coal, wood, charcoal, LPG, and trash also contribute to the emissions of carbon monoxide in urban areas.
Indoors
Indoors and in other closed environments, the concentration of carbon monoxide can quickly and unnoticeably rise to dangerous and fatal levels. The true number of incidents of carbon monoxide poisoning is unknown, since many non-fatal exposures go undetected and unreported. Carbon monoxide poisoning is the most common cause of injury and death due to poisoning worldwide. Poisoning is typically more common during the winter months, probably because of the increased domestic use of gas furnaces, gas or kerosene space heaters, and kitchen stoves during the winter months, which if faulty and/or used without enough ventilation, may produce excessive carbon monoxide.[11][12][13]
In many industrialized countries carbon monoxide is the cause of more than 50 % of fatal poisonings.[14] It has been estimated that more than 40,000 people per year seek medical attention for carbon monoxide poisoning in the United States.[15] Approximately 200 people die each year in the USA from carbon monoxide poisoning caused by home fuel-burning heating equipment.[16] Carbon monoxide poisoning contributes to the approximately 5613 smoke inhalation deaths each year in the United States.[17] The Centers for Disease Control and Prevention (CDC) reports, "Each year, more than 500 Americans die from unintentional carbon monoxide poisoning, and more than 2000 commit suicide by intentionally poisoning themselves."[18]
Uses
Chemical industry
Carbon monoxide gas has many applications in large-scale chemicals manufacturing. Large quantities of aldehydes are produced by the hydroformylation reaction of alkenes, carbon monoxide, and hydrogen. Hydroformylation is coupled to Shell Chemical's Higher Olefin Process (SHOP) to produced detergent precursors.
Methanol is produced by the hydrogenation of carbon monoxide. In a related reaction, the hydrogenation of carbon monoxide is coupled to C-C bond formation, as in the Fischer-Tropsch process which can be used to convert carbon monoxide into liquid hydrocarbon fuels.
In the Monsanto process, carbon monoxide and methanol react in the presence of a rhodium catalyst and hydroiodic acid to produce acetic acid.
Another industrial use for pure carbon monoxide is the Mond process, sometimes called the Carbonyl process, which is utilized to extract and purify of nickel from the raw nickel ore.
Medicine
Following the first report that carbon monoxide is a normal neurotransmitter in 1993,[19] as well as one of three gases that naturally modulate inflammatory responses in the body (the other two being nitric oxide and hydrogen sulphide), carbon monoxide has received a considerable attention as a biological regulator. All three gases are known to act as vasodilators, and encouragers of neovascular growth.[20] However, the issues are complex, as neovascular growth is not always beneficial, since it plays a role in tumor growth, and also the damage from wet macular degeneration, a disease for which smoking (a major source of carbon monoxide in the blood, several times more than natural) increases the risk by a factor of about five.
Many laboratory studies worldwide have been made of the anti-inflammatory and cytoprotective properties of carbon monoxide. These properties have the potential to prevent the development of pathological conditions such as ischemia reperfusion injury, transplant rejection, atherosclerosis, severe sepsis, severe malaria, or autoimmunity. Clinical tests involving humans have been performed, however the results have not yet been released.[21]
Meat coloring
Carbon monoxide is used in modified atmosphere packaging systems in the United States, mainly with fresh meat products to keep them looking fresh. The carbon monoxide combines with myoglobin to form carboxymyoglobin, a bright-cherry-red pigment. This stable red color can persist much longer than in normally packaged meat.[22] Typical levels of carbon monoxide involved in the facilities that use this process are between 0.4 to 0.5 percent by volume.
The technology was first given "generally recognized as safe" (GRAS) status by the U.S. Food and Drug Administration (FDA) in 2002 for use as a secondary packaging system, and does not require labeling. In 2004, the FDA approved the use of carbon monoxide in packaging, declaring that it does not mask any spoilage odor.[23] Despite this ruling, the process remains controversial for fear that it masks spoilage.[24] The process is prohibited in many countries, including Canada, Japan, Singapore and the European Union.
Informational safety resources
- The Invisible Killer, Consumer Product Safety Commission (CPSC).
- Carbon Monoxide Questions and Answers, Consumer Product Safety Commission (CPSC).
- Protect Your Family and Yourself from Carbon Monoxide Poisoning, U.S. Environmental Protection Agency (U.S. EPA).
- Carbon Monoxide Frequently Asked Questions, Centers for Disease Control and Prevention (CDC).
- Exposing an Invisible Killer: The Dangers of Carbon Monoxide, U.S. Fire Administration (USFA) and U.S. Federal Emergency Management Agency (FEMA).
- Carbon Monoxide: OSHA Fact Sheet, U.S. Occupational Safety and Health Administration (OSHA).
- Carbon Monoxide Indoors, American Lung Association.
References
- ↑ Carbon Monoxide - Molecule of the Month, Dr. Mike Thompson, Winchester College, UK.
- ↑ Edward H.Ayres (2009), Crossing the Energy Divide: Moving from Fossil Fuel Dependence to a Clean-Energy Future, Wharton School Publishing, ISBN 0137015445.
- ↑ B. Weinstock and H. Niki (1972), "Carbon Monoxide Balance in Nature", Science, Vol. 176, Issue 4032, pp. 290-292.
- ↑ P. Tikuisis, D.M. Kane, T.M. McLellan, F. Buick and S.M. Fairburn (1992), "Rate of formation of carboxyhemoglobin in exercising humans exposed to carbon monoxide", Journal of Applied Physiology, Vol. 72, Issue 4, pp. 1311-9.
- ↑ Occupational Safety and Health Guideline for Carbon Monoxide
- ↑ Ivan Blumenthal (June 2001), "Carbon monoxide poisoning", Journal of the Royal Society of Medicine, Vol. 94, Issue 6, pp.270-272, available online here.
- ↑ National Carbon Monoxide Emissions by Source Sector, from the website of the U.S. Environmental Protection Agency.
- ↑ Committee on Medical and Biological Effects of Environmental Pollutants (1977), Carbon Monoxide, National Academy of Sciences, page 29, ISBN 0-309-02631-8.
- ↑ An Introduction to Indoor Air Quality: Carbon Monoxide (CO), U.S. Environmental Protection Agency (U.S. EPA).
- ↑ Tom Gosink (1983), What Do Carbon Monoxide Levels Mean?, Alaska Science Forum, Geophysical Institute, University of Alaska.
- ↑ S.R. Thom (October, 2002), "Hyperbaric-oxygen therapy for acute carbon monoxide poisoning", New England Journal of Medicine, Vol. 347, Issue 14, pp. 1105-1106.
- ↑ Armin Ernst and Joseph D. Zibrak (November, 1998), "Carbon monoxide poisoning", New England Journal of Medicine, Vol. 339, Issue 22, pp. 1603-1608.
- ↑ Paul S. Heckerling (May 1987), "Occult carbon monoxide poisoning: a cause of winter headache", American Journal of Emergency Medicine, Vol. 5, Issue 3, pp. 201-204.
- ↑ Stanley T. Omaye (November 2002), "Metabolic modulation of carbon monoxide toxicity", Toxicology, Vol. 180, Issue 2, pp. 139-150.
- ↑ Neil B. Hampson (September 1998), "Emergency department visits for carbon monoxide poisoning in the Pacific Northwest", Journal of Emergency Medicine, Vol. 16, Issue 5, pp.695-698.
- ↑ Carbon Monoxide, Detectors Can Save Lives, CPSC Document #5010, U.S. Consumer Product Safety Commission (CSPC).
- ↑ Nathaniel Cobb and Ruth A. Etzel (August 1991), "Unintentional carbon monoxide-related deaths in the United States, 1979 through 1988", Journal of American Medical Association (JAMA), Vol. 266, Issue 5, pp. 659–663.
- ↑ Carbon Monoxide Poisoning: Questions and Answers, Centers for Disease Control and Prevention (CDC).
- ↑ Carbon Monoxide Gas Is Used by Brain Cells As a Neurotransmitter, Gina Kolata, New York Times article, January 26, 1993.
- ↑ Ling Li, Anna Hsu and Philip K. Moore (2009), "Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation--a tale of three gases!". Pharmacology & Therapeutics, Vol. 123, Issue 3, pp. 386–400.
- ↑ Poison Gas May Carry a Medical Benefit, Carolyn Johnson, Boston Globe article, October 16, 2009.
- ↑ Oddvin Sørheim, Hilde Nissen and Truls Nesbakken (June 1999), "The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide", Journal of Meat Science, Vol. 52, Issue 2, pp. 157–164.
- ↑ S.J. Eilert (September 2005), "New packaging technologies for the 21st century", Journal of Meat Science, Vol. 71, Issue 1, pp. 122–127.
- ↑ Low-Oxygen Packaging With CO: A Study in Food Politics That Warrants Peer Review, Randall D. Huffman and Janet M. Riley, Food Safety Magazine, Dec 2006/Jan 2007.