Methane: Difference between revisions
imported>Yuval Langer m (adding subpages) |
imported>David E. Volk No edit summary |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
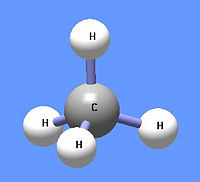
[[Image:Methane DEVolk.jpg|right|thumb|200px|{{#ifexist:Template:Methane DEVolk.jpg/credit|{{Methane DEVolk.jpg/credit}}<br/>|}}Add image caption here.]] | |||
'''Methane''' (CH<sub>4</sub>) is under normal temperature and pressure a colorless and odorless gas, also known as marsh gas. It is lighter than air, boils at −161.4 <sup>0</sup>C and solidifies at | '''Methane''' (CH<sub>4</sub>) is under normal temperature and pressure a colorless and odorless gas, also known as marsh gas. It is lighter than air, boils at −161.4 <sup>0</sup>C and solidifies at | ||
Revision as of 16:05, 4 January 2008
Methane (CH4) is under normal temperature and pressure a colorless and odorless gas, also known as marsh gas. It is lighter than air, boils at −161.4 0C and solidifies at −184 0C. It is highly combustible and forms an explosive mixture with air. The gas is formed by decomposition of plant and animal matter and is the principal component of natural gas and fire damp in coal mines. Methane is used in the heating of homes and the industrial preparation of hydrogen. In chemistry, it is the first member of a series of saturated hydrocarbons, the alkanes. Methane is a potent greenhouse gas. It is believed that there is a large amount of methane contained in water cages (clathrate hydrates) on the ocean floors. Global warming could release this gas and thus enhance considerably the greenhouse effect.