User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok No edit summary |
||
| Line 40: | Line 40: | ||
|} | |} | ||
The molar ratio of methane to product hydrogen (H<sub>2</sub>) in the above cracking reaction is 1 to | The molar ratio of methane to product hydrogen (H<sub>2</sub>) in the above cracking reaction is 1 to 1.5, so 9.351 moles of hydrogen will be formed. Again using the ideal gas law, the volume of hydrogen formed at 0 °C (273.15 K) and 1 atmosphere will be: | ||
:{| border="0" | :{| border="0" | ||
|- | |- | ||
|align=right|<math>V</math> | |align=right|<math>V</math> | ||
|align=left|<math>= \frac{nRT}{P} = \frac{ | |align=left|<math>= \frac{nRT}{P} = \frac{9.351 \cdot 0.08207 \cdot 273.15}{1} = 209.625\ \mbox{L}\ H_2</math> | ||
|} | |} | ||
Since the [[molar mass]]es of acetylene and hydrogen are 26. | Since the [[molar mass]]es of acetylene and hydrogen are 26.036 g/mole and 2.0158 g/mole respectively, the mass of acetylene formed is 26.036 × 3.117 = 81.15 g and the mass of hydrogen formed is 2.0158 × 9.351 = 9.85 g. The total mass of products is then 81.15 + 9.85 = 100.00 g which is equal to the mass of the reactant methane, just as it should be. | ||
Gas stoichiometry sometimes involves having to determine the [[molar mass]] of a gas, given the [[density]] of that gas at a specific temperature and pressure. The ideal gas law can be re-arranged to obtain a relation between the [[density]] and the [[molar mass]] of an ideal gas: | Gas stoichiometry sometimes involves having to determine the [[molar mass]] of a gas, given the [[density]] of that gas at a specific temperature and pressure. The ideal gas law can be re-arranged to obtain a relation between the [[density]] and the [[molar mass]] of an ideal gas: | ||
Revision as of 00:53, 20 June 2010
Gas stoichiometry is the quantitative relationship between the reactants and products in chemical reactions that produce gases. Gas stoichiometry applies when all of the gases involved in a chemical reaction (either reactants or products) may be assumed to be ideal, and the temperature and pressure of the gases are all known. Often, but not always, the reference conditions used for gas stoichiometric calculations are taken to be a temperature of 0 °C and an absolute pressure of 1 atm.
History
In 1792, the German chemist Jeremias Benjamin Richter first proposed the concept of the quantitative mass relationships that exist between the chemicals involved in chemical reactions:
In German: Die stöchyometrie (Stöchyometria) ist die Wissenschaft die quantitativen oder Massenverhältnisse zu messen, in welchen die chymischen Elemente gegen einander stehen." (In English: Stoichiometry is the science of measuring the quantitative proportions or mass ratios in which chemical elements stand to one another.)
He named this quantitative study of chemistry to be "stoichiometry", from two Greek words meaning to measure the magnitude of something that cannot be divided.[1]
Richter's introduction of the term "stoichiometry" substantially predated the introduction of the atomic hypothesis in 1803 by the English eminent scientist John Dalton.
Example calculations
Gas stoichiometry calculations solve for the unknown volume or mass of gaseous products and/or reactants involved in chemical reactions.
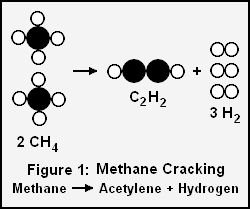
Acetylene and hydrogen produced by cracking methane
For example, if we want to determine the volume of acetylene and hydrogen gases produced by cracking 100 g of methane gas as per this chemical reaction:
- 2 CH4 (g) → C2H2 (g) + 3H2
- 2 moles of methane gas → 1 mole of acetylene gas + 3 moles of hydrogen gas
we would perform the following calculations:
There is a 2 to 1 molar ratio of methane (CH4) to product acetylene (C2H2) in the above cracking reaction, so 3.117 moles of acetylene will be formed. The ideal gas law and the gas law constant of R = 0.08207 L · atm · K−1 · mol−1 can now be used to solve for the volume of acetylene formed at 0 °C (273.15 K) and 1 atmosphere:
The molar ratio of methane to product hydrogen (H2) in the above cracking reaction is 1 to 1.5, so 9.351 moles of hydrogen will be formed. Again using the ideal gas law, the volume of hydrogen formed at 0 °C (273.15 K) and 1 atmosphere will be:
Since the molar masses of acetylene and hydrogen are 26.036 g/mole and 2.0158 g/mole respectively, the mass of acetylene formed is 26.036 × 3.117 = 81.15 g and the mass of hydrogen formed is 2.0158 × 9.351 = 9.85 g. The total mass of products is then 81.15 + 9.85 = 100.00 g which is equal to the mass of the reactant methane, just as it should be.
Gas stoichiometry sometimes involves having to determine the molar mass of a gas, given the density of that gas at a specific temperature and pressure. The ideal gas law can be re-arranged to obtain a relation between the density and the molar mass of an ideal gas:
| where: | |
| = absolute gas pressure | |
| = gas volume | |
| = number of moles | |
| = universal ideal gas law constant | |
| = absolute gas temperature | |
| = gas density at and | |
| = mass of gas | |
| = molar mass of gas |
References
- ↑ Some Stoichiometric Musing] From the website of the [[Illinois State University]'s chemistry department.