User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
<u>'''This needs a lot of | <u>'''This needs a lot of work yet'''</u><BR><BR> | ||
'''Gasoline''' or '''petrol''' is derived from [[petroleum crude oil]]. Conventional gasoline is mostly a blended mixture of more than 200 different [[hydrocarbon]] [[liquid]]s ranging from those containing 4 [[carbon]] [[atom]]s to those containing 11 or 12 carbon atoms. It has an initial [[boiling point]] at [[atmospheric pressure]] of about 35 °[[Celsius|C]] (95 °[[Fahrenheit|F]]) and a final boiling point of about 200 °C (395 °F).<ref name=FAQ>[http://www.faqs.org/faqs/autos/gasoline-faq/part4/ Gasoline FAQ - Part2 of 4], Bruce Hamilton, Industrial Research Ltd. (IRL), a [[Crown Research Institute]] of [[New Zealand]].</ref><ref>{{cite book|author=Gary, J.H. and Handwerk, G.E.|title=Petroleum Refining Technology and Economics|edition=2nd Edition|publisher=Marcel Dekker, Inc.|pages=page 8|year=1984|id=ISBN 0-8247-7150-8}}</ref><ref name=Assi>[http://hqweb.unep.org/pcfv/PDF/JordanWrkshp-Unleaded-Rafat.pdf The Relation Between Gasoline Quality, Octane Number and the Environment], Rafat Assi, National Project Manager of Jordan’s Second National Communications on Climate Change, Presented at Jordan National Workshop on Lead Phase-out, [[United Nations]] Environment Programme, July 2008, [[Amman]], [[Jordan]].</ref><ref>{{cite book|author=James Speight|title=Synthetic Fuels Handbook|edition=1st Edition|publisher=McGraw-Hill|pages=pages 92-93|year=2008|id=ISBN 0-07-149023-X}}</ref> Gasoline is used primarily as fuel for the [[internal combustion engine]]s in automotive vehicles as well in some small airplanes. | '''Gasoline''' or '''petrol''' is derived from [[petroleum crude oil]]. Conventional gasoline is mostly a blended mixture of more than 200 different [[hydrocarbon]] [[liquid]]s ranging from those containing 4 [[carbon]] [[atom]]s to those containing 11 or 12 carbon atoms. It has an initial [[boiling point]] at [[atmospheric pressure]] of about 35 °[[Celsius|C]] (95 °[[Fahrenheit|F]]) and a final boiling point of about 200 °C (395 °F).<ref name=FAQ>[http://www.faqs.org/faqs/autos/gasoline-faq/part4/ Gasoline FAQ - Part2 of 4], Bruce Hamilton, Industrial Research Ltd. (IRL), a [[Crown Research Institute]] of [[New Zealand]].</ref><ref>{{cite book|author=Gary, J.H. and Handwerk, G.E.|title=Petroleum Refining Technology and Economics|edition=2nd Edition|publisher=Marcel Dekker, Inc.|pages=page 8|year=1984|id=ISBN 0-8247-7150-8}}</ref><ref name=Assi>[http://hqweb.unep.org/pcfv/PDF/JordanWrkshp-Unleaded-Rafat.pdf The Relation Between Gasoline Quality, Octane Number and the Environment], Rafat Assi, National Project Manager of Jordan’s Second National Communications on Climate Change, Presented at Jordan National Workshop on Lead Phase-out, [[United Nations]] Environment Programme, July 2008, [[Amman]], [[Jordan]].</ref><ref>{{cite book|author=James Speight|title=Synthetic Fuels Handbook|edition=1st Edition|publisher=McGraw-Hill|pages=pages 92-93|year=2008|id=ISBN 0-07-149023-X}}</ref> Gasoline is used primarily as fuel for the [[internal combustion engine]]s in automotive vehicles as well in some small airplanes. | ||
| Line 35: | Line 35: | ||
*''Isomerate'' (produced in a [[Catalytic isomerization|catalytic isomerization unit]]): has a high content of the branched isomers of pentane and hexane. | *''Isomerate'' (produced in a [[Catalytic isomerization|catalytic isomerization unit]]): has a high content of the branched isomers of pentane and hexane. | ||
== Gasoline | == Gasoline formulations and air quality regulations == | ||
=== In the United States === | |||
There is no "standard" composition or set of specifications for gasoline. In the United States, because of the complex national and individual state and local programs to improve air quality, as well as local refining and marketing decisions, petroleum refiners must supply fuels that meet many different standards. State and local air quality regulations involving gasoline overlap with national requlations and that leads to adjacent or nearby areas having significantly different gasoline specifications. According to a detailed study in 2006, <ref name=CRS>[http://www.scribd.com/doc/1537932/US-Air-Force-rl31361 CRS Report For Congress] ''"Boutique Fuels" and Reformulated Gasoline: Harmonization of Fuel Standards'' (May 10, 2006) , Brent D. Yacobucci, Congressional Research Service, [[Library of Congress]]</ref> there were at least 18 different gasoline formulations required across the United States in 2002. Since many petroleum refiners in the United States produce three grades of fuel and the specifications for fuel marketed in the summer season vary significantly from the specifications in the winter season, that number may have been greatly understated. In any event, the number of fuel formulations has probably increased quite a bit since 2002. In the United States, the various fuel formulations are often referred to as "boutique fuels".<ref name=CRS/><ref>[http://www.epa.gov/oms/boutique.htm Boutique Fuels: State and Local Clean Fuels Programs] From the website of the [[U.S. Environmental Protection Agency]]</ref><ref>[http://www.epa.gov/oms/boutique/420r06901.pdf EPAct Section 1541 Boutique Fuels Report to Congress] Report No. EPA420-R-06-901, December 2006, co-authored by the U.S. Environmental Protection Agency and the [[U.S. Department of Energy]].</ref> | There is no "standard" composition or set of specifications for gasoline. In the United States, because of the complex national and individual state and local programs to improve air quality, as well as local refining and marketing decisions, petroleum refiners must supply fuels that meet many different standards. State and local air quality regulations involving gasoline overlap with national requlations and that leads to adjacent or nearby areas having significantly different gasoline specifications. According to a detailed study in 2006, <ref name=CRS>[http://www.scribd.com/doc/1537932/US-Air-Force-rl31361 CRS Report For Congress] ''"Boutique Fuels" and Reformulated Gasoline: Harmonization of Fuel Standards'' (May 10, 2006) , Brent D. Yacobucci, Congressional Research Service, [[Library of Congress]]</ref> there were at least 18 different gasoline formulations required across the United States in 2002. Since many petroleum refiners in the United States produce three grades of fuel and the specifications for fuel marketed in the summer season vary significantly from the specifications in the winter season, that number may have been greatly understated. In any event, the number of fuel formulations has probably increased quite a bit since 2002. In the United States, the various fuel formulations are often referred to as "boutique fuels".<ref name=CRS/><ref>[http://www.epa.gov/oms/boutique.htm Boutique Fuels: State and Local Clean Fuels Programs] From the website of the [[U.S. Environmental Protection Agency]]</ref><ref>[http://www.epa.gov/oms/boutique/420r06901.pdf EPAct Section 1541 Boutique Fuels Report to Congress] Report No. EPA420-R-06-901, December 2006, co-authored by the U.S. Environmental Protection Agency and the [[U.S. Department of Energy]].</ref> | ||
| Line 57: | Line 49: | ||
=== In other nations === | |||
== Properties that determine the performance of gasolines == | |||
=== Volatility | |||
=== Octane rating === | |||
{{main|Octane rating) | |||
{{Image|Iso-octane and n-Heptane.png|right|250px|Simplified structure of 2,2,4-trimethylpentane and n-heptane.}} | {{Image|Iso-octane and n-Heptane.png|right|250px|Simplified structure of 2,2,4-trimethylpentane and n-heptane.}} | ||
| Line 80: | Line 64: | ||
The octane rating became important in the search for higher output powers from [[aero engine]]s in the late 1930s and the 1940s as it allowed higher [[compression ratio]]s to be used. | The octane rating became important in the search for higher output powers from [[aero engine]]s in the late 1930s and the 1940s as it allowed higher [[compression ratio]]s to be used. | ||
== | === Sulfur content === | ||
=== Stability === | |||
End-product gasoline also contains relatively small amounts of various additives such as antioxidants to improve the gasoline stability during storage by inhibiting the formation of gums, deposit modifiers to reduce engine deposits and fouling, corrosion inhibitors to protect gasoline storage tanks, freezing point depressants to prevent icing, and color dyes for safety or governmental regulatory requirements.<ref name=FAQ/><ref name=Assi/><ref name=Jones>{{cite book|author=David S.J. Jones and Peter P.Pujado (Editors)|title=Handbook of Petroleum Processing|edition=First Edition|publisher=Springer|year=2006|id=ISBN 1-4020-2819-9}}</ref> | |||
{{Image|E10 Water Tolerance.png|right|281px|Temperatures and associated water contents at which a blend of gasoline and 10 volume % ethanol separates.<ref>[http://www.scribd.com/doc/1970087/Environmental-Protection-Agency-Ethanol-Workshop E10 & E85 and Other Alternate Fuels] Bruce Bauman, [[American Petroleum Institute]](API)</ref>}} | |||
When gasoline is left for a period of time, gums and varnishes may build up and precipitate in the gasoline, causing "stale fuel." This will cause gums to build up in the fuel tank, lines, and carburetor or fuel injection components making it harder to start the engine. Motor gasoline may be stored up to 60 days in an approved container. If it is to be stored for a longer period of time, a fuel stabilizer may be used. This will extend the life of the fuel to about 1–2 years, and keep it fresh for the next uses. Fuel stabilizer is commonly used for small engines such as lawnmower and tractor engines to promote quicker and more reliable starting. Users have been advised to keep gasoline containers and tanks more than half full and properly capped to reduce air exposure, to avoid storage at high temperatures,<ref>{{cite web|url=http://www.alpharubicon.com/altenergy/gasstoretg.htm|title=Fuel storage practices}}</ref> to run an engine for ten minutes to circulate the stabilizer through all components prior to storage, and to run the engine at intervals to purge stale fuel from the [[carburetor]].<ref>{{cite web|url=http://www.perr.com/tip1.html|title=PER Notebook}}</ref> | |||
Gummy, sticky resin deposits result from [[oxidation|oxidative]] degradation of gasoline. This degradation can be prevented through the use of [[antioxidant]]s such as [[phenylenediamine]]s, [[alkylenediamine]]s ([[diethylenetriamine]], [[triethylenetetramine]], etc), and [[alkylamine]]s ([[diethylamine]], [[tributylamine]], [[ethylamine]]). Other useful additives include gum inhibitors such as N-substituted [[alkylaminophenol]]s and colour stabilizers such as N-(2-aminoethyl)piperazine, N,N-diethylhydroxylamine, and triethylenetetramine.<ref name=EP0534668>{{patent|EP|0534668|Stabilization of gasoline mixtures}}</ref> | |||
[[ | |||
Improvements in refinery techniques have generally reduced the reliance on the catalytically or thermally cracked stocks most susceptible to oxidation.<ref>{{patent|US|3994698| Gasoline additive concentrate composition}}</ref> Gasoline containing acidic contaminants such as [[naphthenic acid]]s can be addressed with additives including strongly basic organo-amines such as [[N,N-diethylhydroxylamine]], preventing metal corrosion and breakdown of other antioxidant additives due to acidity. Hydrocarbons with a [[bromine number]] of 10 or above can be protected with the combination of unhindered or partially hindered phenols and oil soluble strong amine bases such as [[monoethanolamine]], N-(2-aminoethyl)piperazine, [[cyclohexylamine]], 1,3-cyclohexane-bis(methylamine), 2,5-[[dimethylaniline]], 2,6-dimethylaniline, [[diethylenetriamine]] and [[triethylenetetramine]].<ref name=EP0534668 /> | |||
== Stability == | == Stability == | ||
== References == | == References == | ||
Revision as of 22:54, 2 April 2009
This needs a lot of work yet
Gasoline or petrol is derived from petroleum crude oil. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms to those containing 11 or 12 carbon atoms. It has an initial boiling point at atmospheric pressure of about 35 °C (95 °F) and a final boiling point of about 200 °C (395 °F).[1][2][3][4] Gasoline is used primarily as fuel for the internal combustion engines in automotive vehicles as well in some small airplanes.
In Canada and the United States, the word "gasoline" is commonly used and it is often shortened to simply "gas" although it is a liquid rather than a gas. In fact, gasoline dispensing facilities are referred to as "gas stations".
Most current or former Commonwealth countries use the term "petrol" and dispensing facilities are referred to as "petrol stations". The term "petrogasoline" is also used sometimes. In some European countries and elsewhere, the term "benzin" (or a variant of that word) is used to denote gasoline.
In aviation, "mogas" (short for "motor gasoline") is used to distinguish automotive vehicle fuel from aviation fuel known as "avgas".
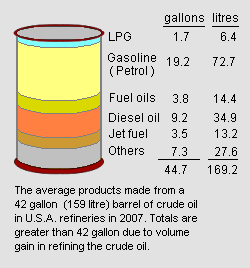
Gasoline production from crude oil
Gasoline and other end-products are produced from petroleum crude oil in petroleum refineries. It is very difficult to quantify the amount of gasoline produced by refining a given amount of crude oil for a number of reasons:
- There are quite literally hundreds of different crude oil sources worldwide and each crude oil has its own unique mixture of thousands of hydrocarbons and other materials.
- There are also hundreds of crude oil refineries worldwide and each of them is designed to process a specific crude oil or a specific set of crude oils. Furthermore, each refinery has its own unique configuration of petroleum refining processes that produces its own unique set of gasoline blend components.
- There are a great many different gasoline specifications that have been mandated by various local, state or national govermental agencies.
- In many geographical areas, the amount of gasoline produced during the summer season (i.e., the season of the greatest demand for automotive gasoline) varies significantly from the amount produced during the winter season.
However, from the data presented in the adjacent image as an average of all the refineries operating in the United States in 2007,[5] refining a barrel of crude oil (i.e., 42 gallons or 159 litres) yielded 19.2 gallons (72.7 litres) of end-product gasoline. That is a volumetric yield of 45.7 percent. The average refinery yield of gasoline in other countries may be different.
From a marketing viewpoint, the most important characteristic of a gasoline is its octane rating (discussed later in this article). Paraffinic hydrocarbons wherein all of the carbon atoms are in a straight chain have the poorest octane ratings. Hydrocarbons with more complicated configurations such as aromatics, olefins and highly branched paraffins have much higher octane ratings. To that end, many of the refining processes used in petroleum refineries are designed to produce hydrocarbons with those more complicated configurations.
Some of the most important refinery process streams that are blended together to obtain the end-product gasolines[6] are:
- Reformate (produced in a catalytic reformer): has a high content of aromatic hydrocarbons and a very low content of olefinic hydrocarbons (alkenes).
- Catalytically cracked gasoline (produced in a fluid catalytic cracker): has a high content of olefinic hydrocarbons and a moderate amount of aromatic hydrocarbons.
- Hydrocrackate (produced in a hydrocracker): has a moderate content of aromatic hydrocarbons.
- Alkylate (produced in an alkylation unit): has a high content of highly branched paraffinic hydrocarbons such as isooctane.
- Isomerate (produced in a catalytic isomerization unit): has a high content of the branched isomers of pentane and hexane.
Gasoline formulations and air quality regulations
In the United States
There is no "standard" composition or set of specifications for gasoline. In the United States, because of the complex national and individual state and local programs to improve air quality, as well as local refining and marketing decisions, petroleum refiners must supply fuels that meet many different standards. State and local air quality regulations involving gasoline overlap with national requlations and that leads to adjacent or nearby areas having significantly different gasoline specifications. According to a detailed study in 2006, [7] there were at least 18 different gasoline formulations required across the United States in 2002. Since many petroleum refiners in the United States produce three grades of fuel and the specifications for fuel marketed in the summer season vary significantly from the specifications in the winter season, that number may have been greatly understated. In any event, the number of fuel formulations has probably increased quite a bit since 2002. In the United States, the various fuel formulations are often referred to as "boutique fuels".[7][8][9]
Some of the major properties and components regulated by the various national and state or local programs are:
- Vapor pressure: The vapor pressure of a gasoline is a measure of its propensity to evaporate. Evaporative emissions of the hydrocarbons in the gasoline lead to the formation of ozone in the atmosphere which reacts with vehicular and industrial emissions of [nitrogen oxides]] (NOx) to form what is called photochemical smog. Smog ias a combination og the words smoke and fog and traditionally referred to the mixture of smoke and sulfur dioxide that resulted from the burning of coal for heating buildings in places such as London, England. Modern photochemical smog does not come from coal burning but from vehicular and industrial emissions of hydrocarbons and nitrogen oxides. It appears as a brownish haze over large urban areas and is irritating to the eyes and lungs.
- Heavy metals:
In other nations
Properties that determine the performance of gasolines
=== Volatility
Octane rating
{{main|Octane rating)
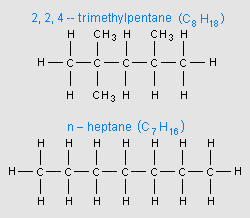
An important characteristic of gasoline is its octane rating, which is a measure of how resistant gasoline is to the abnormal combustion phenomenon known as pre-detonation (also known as knocking, pinging, spark knock, and other names). Deflagration is the normal type of combustion. Octane rating is measured relative to a mixture of 2,2,4-trimethylpentane (an isomer of octane) and n-heptane. There are a number of different conventions for expressing the octane rating; therefore, the same fuel may be labeled with a different number, depending upon the system used.
The octane rating became important in the search for higher output powers from aero engines in the late 1930s and the 1940s as it allowed higher compression ratios to be used.
Sulfur content
Stability
End-product gasoline also contains relatively small amounts of various additives such as antioxidants to improve the gasoline stability during storage by inhibiting the formation of gums, deposit modifiers to reduce engine deposits and fouling, corrosion inhibitors to protect gasoline storage tanks, freezing point depressants to prevent icing, and color dyes for safety or governmental regulatory requirements.[1][3][10]
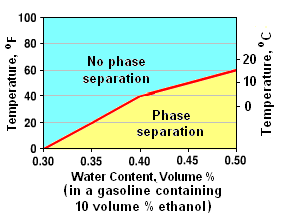
Temperatures and associated water contents at which a blend of gasoline and 10 volume % ethanol separates.[11]
When gasoline is left for a period of time, gums and varnishes may build up and precipitate in the gasoline, causing "stale fuel." This will cause gums to build up in the fuel tank, lines, and carburetor or fuel injection components making it harder to start the engine. Motor gasoline may be stored up to 60 days in an approved container. If it is to be stored for a longer period of time, a fuel stabilizer may be used. This will extend the life of the fuel to about 1–2 years, and keep it fresh for the next uses. Fuel stabilizer is commonly used for small engines such as lawnmower and tractor engines to promote quicker and more reliable starting. Users have been advised to keep gasoline containers and tanks more than half full and properly capped to reduce air exposure, to avoid storage at high temperatures,[12] to run an engine for ten minutes to circulate the stabilizer through all components prior to storage, and to run the engine at intervals to purge stale fuel from the carburetor.[13]
Gummy, sticky resin deposits result from oxidative degradation of gasoline. This degradation can be prevented through the use of antioxidants such as phenylenediamines, alkylenediamines (diethylenetriamine, triethylenetetramine, etc), and alkylamines (diethylamine, tributylamine, ethylamine). Other useful additives include gum inhibitors such as N-substituted alkylaminophenols and colour stabilizers such as N-(2-aminoethyl)piperazine, N,N-diethylhydroxylamine, and triethylenetetramine.[14]
Improvements in refinery techniques have generally reduced the reliance on the catalytically or thermally cracked stocks most susceptible to oxidation.[15] Gasoline containing acidic contaminants such as naphthenic acids can be addressed with additives including strongly basic organo-amines such as N,N-diethylhydroxylamine, preventing metal corrosion and breakdown of other antioxidant additives due to acidity. Hydrocarbons with a bromine number of 10 or above can be protected with the combination of unhindered or partially hindered phenols and oil soluble strong amine bases such as monoethanolamine, N-(2-aminoethyl)piperazine, cyclohexylamine, 1,3-cyclohexane-bis(methylamine), 2,5-dimethylaniline, 2,6-dimethylaniline, diethylenetriamine and triethylenetetramine.[14]
Stability
References
- ↑ 1.0 1.1 Gasoline FAQ - Part2 of 4, Bruce Hamilton, Industrial Research Ltd. (IRL), a Crown Research Institute of New Zealand.
- ↑ Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics, 2nd Edition. Marcel Dekker, Inc., page 8. ISBN 0-8247-7150-8.
- ↑ 3.0 3.1 The Relation Between Gasoline Quality, Octane Number and the Environment, Rafat Assi, National Project Manager of Jordan’s Second National Communications on Climate Change, Presented at Jordan National Workshop on Lead Phase-out, United Nations Environment Programme, July 2008, Amman, Jordan.
- ↑ James Speight (2008). Synthetic Fuels Handbook, 1st Edition. McGraw-Hill, pages 92-93. ISBN 0-07-149023-X.
- ↑ Where Does My Gasoline Come from?, U.S. Department of Energy, Energy Information Administration, April 2008.
- ↑ See the schematic flow diagram in the Petroleum refining processes article.
- ↑ 7.0 7.1 CRS Report For Congress "Boutique Fuels" and Reformulated Gasoline: Harmonization of Fuel Standards (May 10, 2006) , Brent D. Yacobucci, Congressional Research Service, Library of Congress
- ↑ Boutique Fuels: State and Local Clean Fuels Programs From the website of the U.S. Environmental Protection Agency
- ↑ EPAct Section 1541 Boutique Fuels Report to Congress Report No. EPA420-R-06-901, December 2006, co-authored by the U.S. Environmental Protection Agency and the U.S. Department of Energy.
- ↑ David S.J. Jones and Peter P.Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9.
- ↑ E10 & E85 and Other Alternate Fuels Bruce Bauman, American Petroleum Institute(API)
- ↑ Fuel storage practices.
- ↑ PER Notebook.
- ↑ 14.0 14.1 Template:Patent
- ↑ Template:Patent
External links
References
- David S.J. Jones and Peter P.Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9.
- John McKetta (Editor) (1992). Petroleum Processing Handbook. CRC Press. ISBN 0-8247-8681-5.
- Gasoline FAQ - Part2 of 4, Bruce Hamilton, Industrial Research Ltd. (IRL), a Crown Research Institute of New Zealand.
- The Relation Between Gasoline Quality, Octane Number and the Environment, Rafat Assi, National Project Manager of Jordan’s Second National Communications on Climate Change, Presented at Jordan National Workshop on Lead Phase-out, United Nations Environment Programme, July 2008, Amman, Jordan.
- Questions and Answers Relating to the Review of the Existing Fuel Quality Regulations, New Zealand Ministry of Economic Development, December 2005.
- Otto Cycle (About the internal combustion engine four-stroke cycle invented by Nicolaus A. Otto)
- CRS Report For Congress "Boutique Fuels" and Reformulated Gasoline: Harmonization of Fuel Standards (May 10, 2006) , Brent D. Yacobucci, Congressional Research Service, Library of Congress