User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
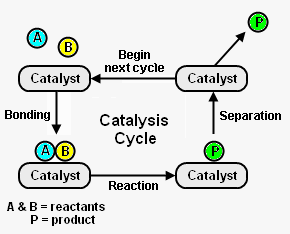
{{Image|Catalysis.png|right|290px| | {{Image|Catalysis.png|right|290px|Figure 1: The catalysis cycle and its elementary steps.}} | ||
'''Catalysis''' is a process that uses a substance to accelerate the rate of a [[chemical reaction]] through an uninterrupted and repeated cycle of elementary steps until the last step regenerates the catalyst in its original form. The substance that does this is known as a '''''catalyst'''''. It is usually present in relatively small amounts and none of it is consumed in the process.<ref name=NAP>{{cite book|author=Commission on Physical Sciences, Mathematics, and Applications (CPSMA), [[National Academies]]|publisher= National Academies Press|title=Catalysis Looks to the Future|edition=|year=1992|id=ISBN 0-309-04584-3}} Available online at [http://www.nap.edu/openbook.php?record_id=1903&page=1 Executive Summary]</ref> | |||
Figure 1 depicts the steps in a typical catalysis cycle. As depicted, the reactant molecules A and B are reacted to yield product P. The catalysis cycle starts with the bonding of reactant molecules A and B to the catalyst. A and B then react to yield product P which is also bound to the catalyst. In the last step, the catalyst is regenerated by product P separating from the catalyst. The regenerated catalyst then begins cycle again by bonding with two more reactant molecules. <ref name=Chork>{{cite book|author=I. Chorkendorff and J. W. Niemantsverdriet|title=Concepts of Modern Catalysts and Kinetics|edition=2nd Edition|publisher=Wiley-VCH|year=2007|id=ISBN 3-527-31672-8}}</ref> | |||
Many substances can act as catalysts, including: [[metal]]s, [[chemical compounds]] (e.g., metal [[oxide]]s, [[sulfide]]s, [[nitride]]s), [[organometallic]] complexes, and [[enzyme]]s. Although a catalyst may be a [[gas]], [[liquid]] or [[solid]], most catalysts used in industrial chemical reactions are in the form of porous pellets. Since not all parts of a solid catalyst participate in the catalysis cycle, those parts that do participate are referred to as ''active sites''. A single porous pellet may have 10<sup>18</sup> active catalytic sites.<ref name=NAP/> | |||
==The catalysis mechanism== | |||
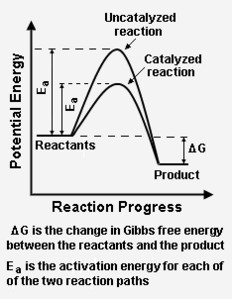
{{Image|Catalysis reaction paths.png|right|232px|Add image caption here.}} | {{Image|Catalysis reaction paths.png|right|232px|Add image caption here.}} | ||
==References== | |||
{{reflist}} | |||
Revision as of 17:16, 6 April 2010
Catalysis is a process that uses a substance to accelerate the rate of a chemical reaction through an uninterrupted and repeated cycle of elementary steps until the last step regenerates the catalyst in its original form. The substance that does this is known as a catalyst. It is usually present in relatively small amounts and none of it is consumed in the process.[1]
Figure 1 depicts the steps in a typical catalysis cycle. As depicted, the reactant molecules A and B are reacted to yield product P. The catalysis cycle starts with the bonding of reactant molecules A and B to the catalyst. A and B then react to yield product P which is also bound to the catalyst. In the last step, the catalyst is regenerated by product P separating from the catalyst. The regenerated catalyst then begins cycle again by bonding with two more reactant molecules. [2]
Many substances can act as catalysts, including: metals, chemical compounds (e.g., metal oxides, sulfides, nitrides), organometallic complexes, and enzymes. Although a catalyst may be a gas, liquid or solid, most catalysts used in industrial chemical reactions are in the form of porous pellets. Since not all parts of a solid catalyst participate in the catalysis cycle, those parts that do participate are referred to as active sites. A single porous pellet may have 1018 active catalytic sites.[1]
The catalysis mechanism
References
- ↑ 1.0 1.1 Commission on Physical Sciences, Mathematics, and Applications (CPSMA), National Academies (1992). Catalysis Looks to the Future. National Academies Press. ISBN 0-309-04584-3. Available online at Executive Summary
- ↑ I. Chorkendorff and J. W. Niemantsverdriet (2007). Concepts of Modern Catalysts and Kinetics, 2nd Edition. Wiley-VCH. ISBN 3-527-31672-8.