imported>Milton Beychok |
imported>Milton Beychok |
| Line 1: |
Line 1: |
| '''Relative volatility''' is a measure that compares the [[vapor pressure]]s of the components in a liquid mixture of chemicals. This measure is widely used in designing large industrial [[distillation]] processes.<ref name=Kister>{{cite book|author=Kister, Henry Z.|title=Distillation Design|edition=1st Edition|publisher=McGraw-hill|year=1992|id=ISBN 0-07-034909-6}}</ref><ref name=Perry>{{cite book|author=Perry, R.H. and Green, D.W. (Editors)|title=Perry's Chemical Engineers' Handbook]edition=7th Edition|publisher=McGraw-hill|year=1997|id=ISBN 0-07-049841-5}}</ref><ref name=SeaderHenley>{{cite book|author= Seader, J. D., and Henley, Ernest J.|title=Separation Process Principles|publisher=Wiley| location=New York|year=1998|id=ISBN 0-471-58626-9}}</ref> In effect, it indicates the ease or difficulty of using distillation to separate the more [[Volatility (chemistry)|volatile]] components from the less volatile components in a mixture. By convention, relative volatility is typically denoted as <math>\alpha</math>. | | [[Image:Total Reflux.png|frame|right|Fractionation at total reflux]] |
| | The '''Fenske equation''' in continuous [[fractional distillation]] is an [[equation]] used for calculating the minimum number of [[Theoretical plate|theoretical plates]] required for the separation of a binary feed stream by a [[Fractionating column|fractionation column]] that is being operated at total [[reflux]] (i.e., which means that no overhead product distillate is being withdrawn from the column). |
|
| |
|
| Relative volatilities are used in the design of all types of distillation processes as well as other [[Separation process|separation]] or [[absorption process]]es that involve the contacting of [[vapor]] and [[liquid]] phases in a series of [[equilibrium stage]]s.
| | The equation was derived by Merrell Fenske in 1932 <ref>Fenske, M.R. (1932). ''Ind.Eng. Chem.'', '''Vol. 24''': 482.</ref>, a professor who served as the head of the [[chemical engineering]] department at the [[Pennsylvania State University]] from 1959 to 1969. |
|
| |
|
| Relative volatilities are not used in separation or absorption processes that involve components that chemically react with each other (for example, the absorption of gaseous [[carbon dioxide]] in aqueous solutions of [[sodium hydroxide]]).
| | This is one of the many different but equivalent versions of the Fenske equation:<ref name=Schreiber>[http://www.eng.fsu.edu/~schreiber/uol/exp300/ Distillation notes] (Loren Schreiber, [[Florida State University]])</ref><ref name=Queens>[http://www.chemeng.queensu.ca/courses/CHEE317/documents/Lecture_13_2007.pdf Lecture 13: Fenske Equation] ([[Queens University]], Canada)</ref><ref>[http://www.chemeng.ed.ac.uk/~jskillin/teaching/sepprocs/2004-05/tutorials/Tut6.pdf Tutorial 6: Separation Processes] (J. Skilling, [[University of Edinburgh]], Scotland)</ref><ref>{{cite book|author=Maxwell, J.B.|title=Data Book on Hydrocarbons|edition=1st Edition|publisher=D. Van Nostrand|year=1950|id=}}</ref><br> |
|
| |
|
| ==Relative volatility for binary liquid mixtures==
| | :<math>\ N = \frac{\log \, \bigg[ \Big(\frac{X_d}{1-X_d}\Big)\Big(\frac{1-X_b}{X_b} \Big) \bigg]}{\log \, \alpha_{avg}} </math> |
| | |
| For a liquid mixture of two components (called a ''binary mixture'') at a given [[temperature]] and [[pressure]], the relative volatility is defined as
| |
| | |
| :<math>\alpha=\frac {(y_i/x_i)}{(y_j/x_j)} = K_i/K_j</math> | |
|
| |
|
| {| border="0" cellpadding="2" | | {| border="0" cellpadding="2" |
| Line 16: |
Line 13: |
| | | | | |
| |- | | |- |
| !align=right|<math>\alpha</math> | | !align=right|<math>N</math> |
| |align=left|= the relative volatility of the more volatile component <math>i</math> to the less volatile component <math>j</math> | | |align=left|= minimum number of theoretical plates required at total reflux (of which the reboiler is one) |
| |- | | |- |
| !align=right|<math>y_i</math> | | !align=right|<math>X_d</math> |
| |align=left|= the [[vapor-liquid equilibrium]] concentration of component <math>i</math> in the vapor phase | | |align=left|= [[mole fraction]] of more [[Volatility (chemistry)|volatile]] component in the overhead distillate |
| |- | |
| !align=right|<math>x_i</math>
| |
| |align=left|= the vapor-liquid equilibrium concentration of component <math>i</math> in the liquid phase
| |
| |- | | |- |
| !align=right|<math>y_j</math> | | !align=right|<math>X_b</math> |
| |align=left|= the vapor-liquid equilibrium concentration of component <math>j</math> in the vapor phase | | |align=left|= mole fraction of more volatile component in the bottoms |
| |-
| |
| !align=right|<math>x_j</math>
| |
| |align=left|= the vapor-liquid equilibrium concentration of component <math>j</math> in the liquid phase
| |
| |- | | |- |
| !align=right|<math>(y/x)</math> | | !align=right|<math>\alpha_{avg}</math> |
| |align=left|= <math>K</math> commonly called the '''''K value''''' or '''''vapor-liquid distribution ratio''''' of a component | | |align=left|= average [[relative volatility]] of more volatile component to less volatile component |
| |} | | |} |
|
| |
|
| When their liquid concentrations are equal, more volatile components have higher vapor pressures than less volatile components. Thus, a <math>K</math> value (= <math>y/x</math>) for a more volatile component is larger than a <math>K</math> value for a less volatile component. That means that <math>\alpha</math> ≥ 1 since the larger <math>K</math> value of the more volatile component is in the numerator and the smaller <math>K</math> of the less volatile component is in the denominator.
| | For ease of expression, the more volatile and the less volatile components are commonly referred to as the '''light key''' (LK) and the '''heavy key''' (HK), respectively. |
|
| |
|
| <math>\alpha</math> is a unitless quantity. When the volatilities of both key components are equal, <math>\alpha</math> = 1 and separation of the two by distillation would be impossible under the given conditions. As the value of <math>\alpha</math> increases above 1, separation by distillation becomes progressively easier. | | If the [[relative volatility]] of the light key to the heavy key is constant from the column top to the column bottom, then <math>\alpha_{avg.}</math> is simply <math>\alpha</math>. If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:<ref name=Schreiber/> |
|
| |
|
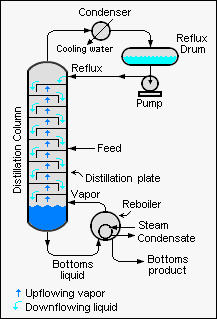
| [[Image:Simple Industrial Distillation Column.png|right|thumb|350px|{{#ifexist:Template:Simple Industrial Distillation Column.png/credit|{{Simple Industrial Distillation Column.png/credit}}<br/>|}}Schematic diagram of a large-scale, simple industrial distillation column.]]
| | :<math>\alpha_{avg.} = \sqrt {(\alpha_t)(\alpha_b)}</math> |
|
| |
|
| When a binary liquid mixture is distilled, complete separation of the two components is rarely achieved. Typically, the overhead fraction from the distillation column consists predominantly of the more volatile component and some small amount of the less volatile component and the bottoms fraction consists predominantly of the less volatile component and some small amount of the more volatile component.
| | {| border="0" cellpadding="2" |
| | |- |
| | |align=right|where: |
| | | |
| | |- |
| | !align=right|<math>\alpha_t</math> |
| | |align=left|= relative volatility of light key to heavy key at top of column |
| | |- |
| | !align=right|<math>\alpha_b</math> |
| | |align=left|= relative volatility of light key to heavy key at bottom of column |
| | |} |
|
| |
|
| ==Relative volatilty for multi-component mixtures==
| | The above Fenske equation can be modified for use in the total reflux distillation of multi-component feeds.<ref name=Queens/><br><br> |
|
| |
|
| A liquid mixture containing many components is called a multi-component mixture. When a multi-component mixture is is distilled, the overhead fraction and the bottoms fraction typically contain much more than one or two components. For example, some intermediate products in a [[Petroleum refining processes|petroleum refinery]] are multi-component liquid mixtures that may contain the [[alkane]], [[alkene]] and [[alkyne]] [[hydrocarbon]]s ranging from [[methane]] having one [[carbon]] [[atom]] to [[decane]]s having ten carbon atoms. For distilling such a mixture, the distillation column may be designed (for example) to produce:
| | ==Another form of the Fenske equation== |
|
| |
|
| * An overhead fraction containing predominantly the more volatile components ranging from methane (having one carbon atom) to [[propane]] (having three carbon atoms)
| | A derivation of another form of the Fenske equation for use in gas chromatography is available on the [[U.S. Naval Academy|U.S. Naval Academy's]] web site.<ref>[http://chemistry.usna.edu/IntegratedLabs/SC263/200603Distillation_06.pdf Fenske Equation] (U.S. Naval Academy)</ref> Using [[Raoult's law]] and [[Dalton's Law]] for a series of condensation and evaporation cycles (i.e., [[equilibrium stage]]s), the following form of the Fenske equation is obtained: |
| * A bottoms fraction containing predominantly the less volatile components ranging from [[isobutane]] (having four carbon atoms) to decanes (ten carbon atoms).
| |
|
| |
|
| Such a distillation column is typically called a ''depropanizer''. The column designer would designate the key components governing the separation design to be propane as the so-called '''light key (LK)''' and isobutane as the so-called ''' heavy key (HK)'''. In that context, a lighter component means a component with a lower [[boiling point]] (or a higher vapor pressure) and a heavier component means a component with a higher boiling point (or a lower vapor pressure).
| | :<math>\ \frac{Z_a}{Z_b} = \frac{X_a}{X_b} \left (\frac{P^0_a}{P^0_b} \right) ^N </math> |
|
| |
|
| Thus, for the distillation of any multi-component mixture, the relative volatility is often defined as
| | {| border="0" cellpadding="2" |
| | | |- |
| :<math>\alpha=\frac {(y_{LK}/x_{LK})}{(y_{HK}/x_{HK})} = K_{LK}/K_{HK}</math>
| | |align=right|where: |
| | | | |
| Large-scale industrial distillation is rarely undertaken if the relative volatility is less than 1.05.<ref name=Perry/>
| | |- |
| | | !align=right|<math>N</math> |
| The values of <math>K</math> have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.<ref>DePriester, C.L. (1953), ''Chem. Eng. Prog. Symposium Series'', 7, '''49''', pages 1-43</ref><ref>[http://www.utwired.engr.utexas.edu/che363/docs/abs/abs1a_doc.pdf DePriester Charts]</ref>
| | |align=left|= number of equilibrium stages |
| | | |- |
| <math>K</math> values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in petroleum refineries, [[petrochemical]] and [[chemical plant]]s, [[natural gas processing]] plants and other industries. | | !align=right|<math>Z_n</math> |
| | | |align=left|= mole fraction of component n in the vapor phase |
| ==Effect of temperature or pressure on relative volatility== | | |- |
| An increase in pressure has a significant effect on the relative volatility of the components in a liquid mixture. Since an increase in the pressure requires an increase in the temperature, then an increase in temperature also effects the relative volatility.
| | !align=right|<math>X_n</math> |
| | | |align=left|= mole fraction of component n in the liquid phase |
| The diagram below is based on the vapor-liquid equilibrium of a hypothetical binary liquid mixture and illustrates how an increase in either the pressure or temperature decreases the relative volatility of the mixture.
| | |- |
| | | !align=right|<math>{P^0_n}</math> |
| [[Image:Relative Volatility vsT&P.png|center|thumb|374px|{{#ifexist:Template:Relative Volatility vsT&P.png/credit|{{Relative Volatility vsT&P.png/credit}}<br/>|}}]]
| | |align=left|= [[vapor pressure]] of pure component n |
| | |} |
|
| |
|
| ==References== | | ==References== |
| {{Reflist}} | | {{reflist}} |
Fractionation at total reflux
The Fenske equation in continuous fractional distillation is an equation used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a fractionation column that is being operated at total reflux (i.e., which means that no overhead product distillate is being withdrawn from the column).
The equation was derived by Merrell Fenske in 1932 [1], a professor who served as the head of the chemical engineering department at the Pennsylvania State University from 1959 to 1969.
This is one of the many different but equivalent versions of the Fenske equation:[2][3][4][5]
![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {X_{d}}{1-X_{d}}}{\Big )}{\Big (}{\frac {1-X_{b}}{X_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/06af3d7fcd0e4cac0751fb951d065cca1c4fcaf5)
| where:
|
|

|
= minimum number of theoretical plates required at total reflux (of which the reboiler is one)
|

|
= mole fraction of more volatile component in the overhead distillate
|

|
= mole fraction of more volatile component in the bottoms
|

|
= average relative volatility of more volatile component to less volatile component
|
For ease of expression, the more volatile and the less volatile components are commonly referred to as the light key (LK) and the heavy key (HK), respectively.
If the relative volatility of the light key to the heavy key is constant from the column top to the column bottom, then  is simply
is simply  . If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:[2]
. If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:[2]

| where:
|
|

|
= relative volatility of light key to heavy key at top of column
|

|
= relative volatility of light key to heavy key at bottom of column
|
The above Fenske equation can be modified for use in the total reflux distillation of multi-component feeds.[3]
Another form of the Fenske equation
A derivation of another form of the Fenske equation for use in gas chromatography is available on the U.S. Naval Academy's web site.[6] Using Raoult's law and Dalton's Law for a series of condensation and evaporation cycles (i.e., equilibrium stages), the following form of the Fenske equation is obtained:

| where:
|
|

|
= number of equilibrium stages
|

|
= mole fraction of component n in the vapor phase
|

|
= mole fraction of component n in the liquid phase
|

|
= vapor pressure of pure component n
|
References

![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {X_{d}}{1-X_{d}}}{\Big )}{\Big (}{\frac {1-X_{b}}{X_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/06af3d7fcd0e4cac0751fb951d065cca1c4fcaf5)












