User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
'''Relative volatility''' is a measure that compares the [[vapor pressure]]s of the components in a liquid mixture of chemicals. This measure is widely used in designing large industrial [[distillation]] processes.<ref name=Kister>{{cite book|author=Kister, Henry Z.|title= | '''Relative volatility''' is a measure that compares the [[vapor pressure]]s of the components in a liquid mixture of chemicals. This measure is widely used in designing large industrial [[distillation]] processes.<ref name=Kister>{{cite book|author=Kister, Henry Z.|title=Distillation Design|edition=1st Edition|publisher=McGraw-hill|year=1992|id=ISBN 0-07-034909-6}}</ref><ref name=Perry>{{cite book|author=Perry, R.H. and Green, D.W. (Editors)|title=Perry's Chemical Engineers' Handbook]edition=7th Edition|publisher=McGraw-hill|year=1997|id=ISBN 0-07-049841-5}}</ref><ref name=SeaderHenley>{{cite book|author= Seader, J. D., and Henley, Ernest J.|title=Separation Process Principles|publisher=Wiley| location=New York|year=1998|id=ISBN 0-471-58626-9}}</ref> In effect, it indicates the ease or difficulty of using distillation to separate the more [[Volatility (chemistry)|volatile]] components from the less volatile components in a mixture. By convention, relative volatility is typically denoted as <math>\alpha</math>. | ||
Relative volatilities are used in the design of all types of distillation processes as well as other [[Separation process|separation]] or absorption | Relative volatilities are used in the design of all types of distillation processes as well as other [[Separation process|separation]] or [[absorption process]]es that involve the contacting of [[vapor]] and [[liquid]] phases in a series of [[equilibrium stage]]s. | ||
Relative volatilities are not used in separation or absorption processes that involve components | Relative volatilities are not used in separation or absorption processes that involve components that chemically react with each other (for example, the absorption of gaseous [[carbon dioxide]] in aqueous solutions of [[sodium hydroxide]]). | ||
== | ==Relative volatility for binary liquid mixtures== | ||
For a liquid mixture of two components (called a ''binary mixture'') at a given [[temperature]] and [[pressure]], the relative volatility is defined as | For a liquid mixture of two components (called a ''binary mixture'') at a given [[temperature]] and [[pressure]], the relative volatility is defined as | ||
| Line 41: | Line 41: | ||
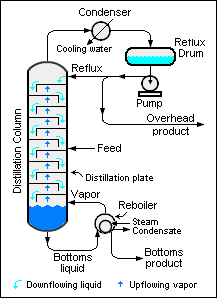
[[Image:Simple Industrial Distillation Column.png|right|thumb|350px|{{#ifexist:Template:Simple Industrial Distillation Column.png/credit|{{Simple Industrial Distillation Column.png/credit}}<br/>|}}Schematic diagram of a large-scale, simple industrial distillation column.]] | [[Image:Simple Industrial Distillation Column.png|right|thumb|350px|{{#ifexist:Template:Simple Industrial Distillation Column.png/credit|{{Simple Industrial Distillation Column.png/credit}}<br/>|}}Schematic diagram of a large-scale, simple industrial distillation column.]] | ||
When a binary liquid mixture is distilled, complete separation of the two components is rarely achieved. Typically, the overhead fraction from the distillation column consists predominantly of the more volatile component and some small amount of the less volatile component and the bottoms fraction consists predominantly of the less volatile component and some small amount of the more volatile component. | |||
A liquid mixture containing many components is called a multi-component mixture. When a multi-component mixture is is distilled, the overhead fraction and the bottoms fraction typically contain much more than one or two components. For example, some intermediate products in | ==Relative volatilty for multi-component mixtures== | ||
A liquid mixture containing many components is called a multi-component mixture. When a multi-component mixture is is distilled, the overhead fraction and the bottoms fraction typically contain much more than one or two components. For example, some intermediate products in a [[Petroleum refining processes|petroleum refinery]] are multi-component liquid mixtures that may contain the [[alkane]], [[alkene]] and [[alkyne]] [[hydrocarbon]]s ranging from [[methane]] having one [[carbon]] [[atom]] to [[decane]]s having ten carbon atoms. For distilling such a mixture, the distillation column may be designed (for example) to produce: | |||
* An overhead fraction containing predominantly the more volatile components ranging from methane (having one carbon atom) to [[propane]] (having three carbon atoms) | * An overhead fraction containing predominantly the more volatile components ranging from methane (having one carbon atom) to [[propane]] (having three carbon atoms) | ||
* A bottoms fraction containing predominantly the less volatile components ranging from [[isobutane]] (having four carbon atoms) to decanes (ten carbon atoms). | * A bottoms fraction containing predominantly the less volatile components ranging from [[isobutane]] (having four carbon atoms) to decanes (ten carbon atoms). | ||
Such a distillation column is typically called a depropanizer. The designer would designate the key components governing the separation design to be propane as the so-called '''light key (LK)''' and isobutane as the so-called ''' heavy key (HK)'''. In that context, a lighter component means a component with a lower [[boiling point]] (or a higher vapor pressure) and a heavier component means a component with a higher boiling point (or a lower vapor pressure). | Such a distillation column is typically called a ''depropanizer''. The column designer would designate the key components governing the separation design to be propane as the so-called '''light key (LK)''' and isobutane as the so-called ''' heavy key (HK)'''. In that context, a lighter component means a component with a lower [[boiling point]] (or a higher vapor pressure) and a heavier component means a component with a higher boiling point (or a lower vapor pressure). | ||
Thus, for the distillation of any multi-component mixture, the relative volatility is often defined as | Thus, for the distillation of any multi-component mixture, the relative volatility is often defined as | ||
| Line 58: | Line 60: | ||
The values of <math>K</math> have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.<ref>DePriester, C.L. (1953), ''Chem. Eng. Prog. Symposium Series'', 7, '''49''', pages 1-43</ref><ref>[http://www.utwired.engr.utexas.edu/che363/docs/abs/abs1a_doc.pdf DePriester Charts]</ref> | The values of <math>K</math> have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.<ref>DePriester, C.L. (1953), ''Chem. Eng. Prog. Symposium Series'', 7, '''49''', pages 1-43</ref><ref>[http://www.utwired.engr.utexas.edu/che363/docs/abs/abs1a_doc.pdf DePriester Charts]</ref> | ||
<math>K</math> values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in | <math>K</math> values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in petroleum refineries, [[petrochemical]] and [[chemical plant]]s, [[natural gas processing]] plants and other industries. | ||
==References== | ==References== | ||
{{Reflist}} | {{Reflist}} | ||
Revision as of 20:00, 16 February 2008
Relative volatility is a measure that compares the vapor pressures of the components in a liquid mixture of chemicals. This measure is widely used in designing large industrial distillation processes.[1][2][3] In effect, it indicates the ease or difficulty of using distillation to separate the more volatile components from the less volatile components in a mixture. By convention, relative volatility is typically denoted as .
Relative volatilities are used in the design of all types of distillation processes as well as other separation or absorption processes that involve the contacting of vapor and liquid phases in a series of equilibrium stages.
Relative volatilities are not used in separation or absorption processes that involve components that chemically react with each other (for example, the absorption of gaseous carbon dioxide in aqueous solutions of sodium hydroxide).
Relative volatility for binary liquid mixtures
For a liquid mixture of two components (called a binary mixture) at a given temperature and pressure, the relative volatility is defined as
| where: | |
| = the relative volatility of the more volatile component to the less volatile component | |
| = the vapor-liquid equilibrium concentration of component in the vapor phase | |
| = the vapor-liquid equilibrium concentration of component in the liquid phase | |
| = the vapor-liquid equilibrium concentration of component in the vapor phase | |
| = the vapor-liquid equilibrium concentration of component in the liquid phase | |
| = commonly called the K value or vapor-liquid distribution ratio of a component |
When their liquid concentrations are equal, more volatile components have higher vapor pressures than less volatile components. Thus, a value (= ) for a more volatile component is larger than a value for a less volatile component. That means that ≥ 1 since the larger value of the more volatile component is in the numerator and the smaller of the less volatile component is in the denominator.
is a unitless quantity. When the volatilities of both key components are equal, = 1 and separation of the two by distillation would be impossible under the given conditions. As the value of increases above 1, separation by distillation becomes progressively easier.
When a binary liquid mixture is distilled, complete separation of the two components is rarely achieved. Typically, the overhead fraction from the distillation column consists predominantly of the more volatile component and some small amount of the less volatile component and the bottoms fraction consists predominantly of the less volatile component and some small amount of the more volatile component.
Relative volatilty for multi-component mixtures
A liquid mixture containing many components is called a multi-component mixture. When a multi-component mixture is is distilled, the overhead fraction and the bottoms fraction typically contain much more than one or two components. For example, some intermediate products in a petroleum refinery are multi-component liquid mixtures that may contain the alkane, alkene and alkyne hydrocarbons ranging from methane having one carbon atom to decanes having ten carbon atoms. For distilling such a mixture, the distillation column may be designed (for example) to produce:
- An overhead fraction containing predominantly the more volatile components ranging from methane (having one carbon atom) to propane (having three carbon atoms)
- A bottoms fraction containing predominantly the less volatile components ranging from isobutane (having four carbon atoms) to decanes (ten carbon atoms).
Such a distillation column is typically called a depropanizer. The column designer would designate the key components governing the separation design to be propane as the so-called light key (LK) and isobutane as the so-called heavy key (HK). In that context, a lighter component means a component with a lower boiling point (or a higher vapor pressure) and a heavier component means a component with a higher boiling point (or a lower vapor pressure).
Thus, for the distillation of any multi-component mixture, the relative volatility is often defined as
Large-scale industrial distillation is rarely undertaken if the relative volatility is less than 1.05.[2]
The values of have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.[4][5]
values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in petroleum refineries, petrochemical and chemical plants, natural gas processing plants and other industries.
References
- ↑ Kister, Henry Z. (1992). Distillation Design, 1st Edition. McGraw-hill. ISBN 0-07-034909-6.
- ↑ 2.0 2.1 Perry, R.H. and Green, D.W. (Editors) (1997). Perry's Chemical Engineers' Handbook]edition=7th Edition. McGraw-hill. ISBN 0-07-049841-5.
- ↑ Seader, J. D., and Henley, Ernest J. (1998). Separation Process Principles. New York: Wiley. ISBN 0-471-58626-9.
- ↑ DePriester, C.L. (1953), Chem. Eng. Prog. Symposium Series, 7, 49, pages 1-43
- ↑ DePriester Charts