Ketoconazole: Difference between revisions
imported>David E. Volk |
imported>David E. Volk (→Conditions with increased risks: ergotamines) |
||
| Line 22: | Line 22: | ||
=== Conditions with increased risks === | === Conditions with increased risks === | ||
The risk of [[myopathy]] and [[rhabdomyolysis]] is increased when ketoconazole is taken with some cholesterol-lowering [[statin]] drugs such as [[atorvastatin]], [[cerivastatin]], [[lovastatin]] and [[simvastatin]]. The risk of [[arrhythmia]]s and [[cardiotoxicity]] increase when this drug is taken with [[astemizole]], [[cisapride]], [[terfenadine]], [[pimozide]]. | The risk of [[myopathy]] and [[rhabdomyolysis]] is increased when ketoconazole is taken with some cholesterol-lowering [[statin]] drugs such as [[atorvastatin]], [[cerivastatin]], [[lovastatin]] and [[simvastatin]]. The risk of [[arrhythmia]]s and [[cardiotoxicity]] increase when this drug is taken with [[astemizole]], [[cisapride]], [[terfenadine]], [[pimozide]]. An increased risk of ergotism and severe ischemia may result when ketoconazole is taken with [[ergotamine]] and its derivate [[dihydroergotamine]]. | ||
=== Drugs which decrease the effectiveness of ketoconazole === | === Drugs which decrease the effectiveness of ketoconazole === | ||
Revision as of 13:36, 6 March 2008
Ketoconazole is one of the azole-based antifungal drugs used to treat fungal infections. Its use has greatly diminished because of the introduction of better treatment alternatives, including the triazoles fluconazole and itraconazole. It is based on imidazole as are clotrimazole, fluconazole, itraconazole and miconazole. It is a second-line of defense systemic drug use to treat candidiasis, candiduria, oral thrush, mucocutaneious candidiasis, blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis and paracocccidioidomycosis.
Mechanism of action
Azole-based antifungal agents, such as ketoconazole, work by inhibiting the enzyme cytochrome P450 14--demethylase (P45014DM), which is part of the sterol biosynthesis pathway that converts lanosterol to ergosterol[1]. Because ketoconazole has less affinity towards fungal cell membranes than the newer triazole antifungal agents like fluconazole and itraconazole, it is more likely to bind with mammalian cell membranes and induce toxicity[2]. .
Side effects
Becuase ketoconazole disrupts part of the sterol biosynthesis pathway, it may decrease levels of the steroids testosterone and cortisone causing gynecomastia and oligospermia in males and irregular menstration in women. It may also cause anorexia, nausea and vomiting, increased levels of transaminase and liver toxicity.
Chemistry
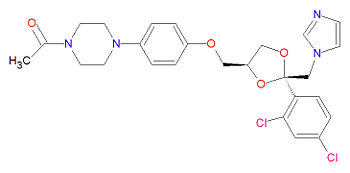
The IUPAC chemical name for ketoconazole is 1-[4-[4-[[2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone. It has chemical formula C26H28Cl2N4O4, a molecular mass of 531.4309 g/mol and is registered under CAS Number 79156-75-5.
Drug interactions
Drug with enhanced effectiveness when taken with ketoconazole
Ketoconazole increases the effectiveness, and therefore toxicity of many medications. It increases the effectiveness of some anticoagualant medications, including acenocoumarol, anisindione, dicumarol and warfarin and the effectiveness and toxicity of some steroids and steroid agonists, including the corticosteroids methylprednisolone, prednisolone and prednisone, the glucocorticoids budesonide and ciclesonide and the aldosterone agonist eplerenone. It also increase the effects of some antiviral drugs, including indinavir, ritonavir and saquinavir. Many benzodiazepine-based drugs, including alazepam, alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, midazolam, quazepam and triazolam are more effective when taken with ketoconazole. The immunosuppressants cyclosporine, everolimus, sirolimus and tacrolimus, and the tricyclic drugs amitriptyline, imipramine and nortriptyline, are enhanced when taken with ketoconazole. Because ketoconazole is a potent CYP3A4 inhibitor, it increases the effects and toxicity of almotriptan, aprepitant, dofetilide, eletriptan, erlotinib, gefitinib, trazodone, and also slows the metabolism of darifenacin and solifenacin. Drugs used to treat erectile dysfunction, like sildenafil (Viagra®, Revatio®), tadalafil (Cialis®) and vardenafil (Levitra®), and drugs used to treat diabetes, such as pioglitazone, rosiglitazone and Tolbutamide have increased effects when taken with ketoconazole. The psycholeptic drugs aripiprazole, buspirone, galantamine, haloperidol, quetiapine and ziprasidone are likewise more effective.
Drugs with decreased effectiveness when taken with ketoconazole
Some oral contraceptives, including ethinyl estradiol and mestranol may be less effective when taken in combination with ketoconazole.
Conditions with increased risks
The risk of myopathy and rhabdomyolysis is increased when ketoconazole is taken with some cholesterol-lowering statin drugs such as atorvastatin, cerivastatin, lovastatin and simvastatin. The risk of arrhythmias and cardiotoxicity increase when this drug is taken with astemizole, cisapride, terfenadine, pimozide. An increased risk of ergotism and severe ischemia may result when ketoconazole is taken with ergotamine and its derivate dihydroergotamine.
Drugs which decrease the effectiveness of ketoconazole
Antacids, including basic forms of aluminium, bismuth, calcium and magnesium , as well as the proton pump inhibitors esomeprazole, lansoprazole, omeprazole, pantoprazole and rabeprazole decrease the absorption of imidazole and hence make ketoconazole less effective. Likewise, the anti-H2 drugs cimetidine, famotidine, nizatidine and ranitidine supress ketoconazole absorption. Additional medications with this side effect include isoniazid, nevirapine, rifampin and sucralfate.
Note: This section is still in progress
Synonyms
- (+-)-cis-1-acetyl-4-(p-((2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazine,
- 79156-75-5
- CPD-4503
- fungarest
- fungoral,
- ketoconazol,
- ketoconazole
- ketoderm
- ketoisdin,
- KW 1414
- NCI60_002728
- nizoral
- NSC317629
- orifungal M
- panfungol
Brand names
- Extina®
- Fungarest®
- Fungoral®
- Ketocanazole®
- Ketoconazol®
- Ketoconazol®
- Ketoconazole®
- Ketoconazolum®
- Ketoderm®
- Ketoisdin®
- Ketozole®
- Nizoral®
- Nizoral Cream®
- Nizoral Shampoo®
- Nizoral® A-D
- Nizoral® A-D Shampoo
- Orifungal®
- Orifungal M®
- Panfungol®
External links
- Doctorfungus [1]
- Ketoconazole - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank