Genetics of obesity: Difference between revisions
imported>Ailsa Oswald |
imported>Ailsa Oswald |
||
| Line 39: | Line 39: | ||
===Mendelian Disorders=== | ===Mendelian Disorders=== | ||
Obesity is a major clinical feature (but not the dominant feature) of some mendelian disorders for which genetic mutations have been found. ( | Obesity is a major clinical feature (but not the dominant feature) of some mendelian disorders for which genetic mutations have been found. <ref name=Loos RJ, Bouchard C 2003> Loos RJ, Bouchard C. Obesity--is it a genetic disorder? J Intern Med. 2003 Nov;254(5):401-25. PMID 14535962 </ref> | ||
Prader-Willi syndrome is autosomal dominant and characterised by obesity, reduced foetal activity, mental retardation and hyperphagia which develops between the age of 1 and 2. Around three quarters of patients have a deletion on chromosome 15 (15q11-q13) and the remaining have maternal disomy. Albright hereditary osteodystrophy is a rare autosomal dominant disorder where patients are obese, short, mentally retarded and have subcutaneous calcifications. AHO is due to parental imprinting of mutations in the GNAS1 gene. | Prader-Willi syndrome is autosomal dominant and characterised by obesity, reduced foetal activity, mental retardation and hyperphagia which develops between the age of 1 and 2. Around three quarters of patients have a deletion on chromosome 15 (15q11-q13) and the remaining have maternal disomy. Albright hereditary osteodystrophy is a rare autosomal dominant disorder where patients are obese, short, mentally retarded and have subcutaneous calcifications. AHO is due to parental imprinting of mutations in the GNAS1 gene. <ref name=Loos RJ, Bouchard C 2003/> | ||
The mutations for these have been established but, despite attempts, there has been no link between mutant genes and disrupted energy balance. It is likely that the underlying genetics that cause obesity in these mendelian disorders are different to those in common forms of obesity, though further studies investigating genetic markers would need to be carried out. | The mutations for these have been established but, despite attempts, there has been no link between mutant genes and disrupted energy balance. It is likely that the underlying genetics that cause obesity in these mendelian disorders are different to those in common forms of obesity, though further studies investigating genetic markers would need to be carried out. <ref name=Loos RJ, Bouchard C 2003/> | ||
===Single-gene disorders=== | ===Single-gene disorders=== | ||
Revision as of 15:45, 9 November 2010
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 01 February 2011. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
Begin your article with a brief overview of the scope of the article. Include the article name Genetics of Obesity Remember you are writing an encyclopedia article; it is meant to be readable by a wide audience, and so you will need to explain some things clearly, without using unneccessary jargon.
Overview
Obesity is the condition of excessive fat accummulation typically defined as a BMI of 30 or more. It presents as a major risk factor for premature mortality and is attributable to a number of chronic diseases: cardiovascular, metabolic and cancerous [1]. With the onset of the obesity epidemic, an increasing number of institutions are researching into the causes of this current trend. While the environmental influences (including the ease of access to high energy palatable foods and sedentary lifestyle) cannot be neglected, there is much focus on genomics to explain inter-individual variation in susceptibility to adiposity (2).
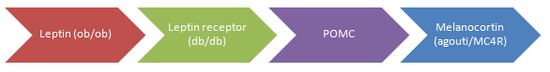
Although several genes have been identified, they are limited to monogenic causes. Many of these are mutations of proteins in the “leptin pathway” which has an important role in energy balance. Each of those represented in the diagram has been shown to cause obesity, most often through twin studies.
In the case of all (except for MC4R) the quantity of mutations remains insignificant in the average population. However these findings do suggest there can be more common multifactorial influences on susceptibility to adiposity which are likely to be involved in similar pathways (7).
Population genetics
Pima Indians
The Pima Indian population represents a useful model for demonstrating the potential impact of genetic factors in influencing obesity. The Pima once resided in the deserts of Mexico where resources were scarce, however some migrated and communities now exist in Arizona, USA. Those who settled in the US were presented with a relative abundance of nourishment and exhibit extremely high levels of obesity and type 2 diabetes, whereas other communities of Pima do not. It has been proposed that genetic factors have protected this population in the past and allowed them to withstand conditions of deprivation, though if exposed to greater levels of food intake they are more susceptible to developing obesity as a result. This is known as the 'thrifty gene hypothesis'. [8]
Thrifty gene hypothesis
The thrifty gene hypothesis was first put forward by Neel in 1962[2] It states that, in history, a genotype that stores energy more efficiently in times of food abundance would have been advantageous to our ancestors to survive times of food shortage. It is widely accepted that this genotype has been naturally selected through years of food shortage but in modern day society has become a source health problems. These days food is almost always easily available so those showing the thrifty phenotype are in constant food storage mode preparing their bodies for a period of food shortage that never comes. This is suggested to be causing the widespread prevalence of obesity and type 2 diabetes in the developed world.
More recently this hypothesis has been challenged. Most noteably, JR Speakman highlighted some problems of the thrifty gene hypothesis in a review for the International Society of Diabetes Vascular Disease. He suggested that not enough significant famines have occurred in human populations and that mortality levels during these famines would not have been sufficient to lead to the levels of natural genotype selection that the thrifty gene hypothesis implies. Mortality patterns also do not fit with the hypothesis because deaths were often not due to starvation but due to disease, so the thrifty phenotype would not have been particularly advantageous and those age groups incurring the highest mortality rates would have been the very young and very old, and not those of reproductive age so gene selection for future generations would be unaffected. Whether the thrifty geneotype explains some of modern day obesity remains unclear as records of famine and mortality rates in history are not always well kept.
Polynesian Populations
Polynesia is a subregion of Oceania, encompassing more than 1000 islands over the central and southern Pacific Ocean. Polynesians share language, culture, beliefs and other features of society. The populations are interesting for study because of the relatively conserved gene pool, the concept of modernisation, and the migration of Polynesians to other countries. Polynesian populations exhibit high rates of type 2 diabetes and obesity, as shown in Samoans by McGarvey.[3] However, increased rates of other obesity-associated problems such as metabolic syndrome and dyslipidaemia are not observed.[4] Finally, a study in several Oceanic populations did not support Neel's thrifty gene hypothesis: population frequencies of common FTO polymorphisms displayed no significant association with BMI.[5]
Monozygotic Twin Studies
Twin studies are useful for reliable investigation of the gene-environment interactions of obesity as they can yield more powerful data [6] Using twins in a study by Bouchard et al [7] shows whether the already known inter-individual effects of changes in energy balance or dietary interventions are due to genetic factors. For example, exposing monozygotic twins to positive energy balance/overfeeding lets us investigate whether differing sensitivities in individuals gaining fat when exposed to positive energy balance is dependent on genotype or not. Variables in phenotype measured in the study included; body weight, body composition, fat distribution, abdominal visceral fat and resting metabolic rate. The findings after overfeeding/exercise in the twins conveyed clear intrapair resemblance and variation between different twin pairs. This suggested the differences in susceptibility of overeating must be controlled mainly by genetic factors (which are thought to be inherited), though the exact genes involved in sensitivity of energy balance are currently not known. [7]
Using monozygotic twin studies also allows measurement of the relationship between dietary factors and body fat independent of genetic factors. [8] Both diet and genetic influences are thought to influence body fat. Using monozygotic twin studies it is possible to investigate the effect of diet on body fat independent of genotype. This therefore lets us analyse the extent genetics are involved in influencing body fat as no relationship between dietary fat and body fat was found in middle-aged women in a study conducted by Samaras et al [9], indicating that diet in determining total body fat may have been overestimated in the past and genetic factors are perhaps therefore more influential.
Monogenetic forms of Obesity
Mendelian Disorders
Obesity is a major clinical feature (but not the dominant feature) of some mendelian disorders for which genetic mutations have been found. Cite error: Invalid <ref> tag; invalid names, e.g. too many
Prader-Willi syndrome is autosomal dominant and characterised by obesity, reduced foetal activity, mental retardation and hyperphagia which develops between the age of 1 and 2. Around three quarters of patients have a deletion on chromosome 15 (15q11-q13) and the remaining have maternal disomy. Albright hereditary osteodystrophy is a rare autosomal dominant disorder where patients are obese, short, mentally retarded and have subcutaneous calcifications. AHO is due to parental imprinting of mutations in the GNAS1 gene. Cite error: Invalid <ref> tag; invalid names, e.g. too many
The mutations for these have been established but, despite attempts, there has been no link between mutant genes and disrupted energy balance. It is likely that the underlying genetics that cause obesity in these mendelian disorders are different to those in common forms of obesity, though further studies investigating genetic markers would need to be carried out. Cite error: Invalid <ref> tag; invalid names, e.g. too many
Single-gene disorders
Leptin
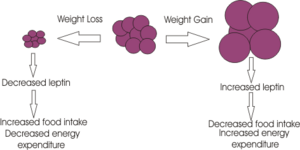
Leptin is an endocrine hormone released from the adipocytes which allows information regarding energy stores to be transmitted to the brain, primarily the hypothalamus. It inhibits appetite by inhibiting the release of NYP and it stimulates metabolic rate. The levels circulating in the blood are proportional to amount of adipose tissue. (2)
There have been a few patients worldwide found for leptin mutations. Montague et al (3) investigated two morbidly obese hyperphagic children that were cousins with undetectable leptin. They were found to both be homozygous for deletion of a single guanine on codon 133, leading to a premature stop codon. The children had a strong resemblance to the ob/ob mice which are obese, hyperphagic, infertile and have hyperinsulinaemia. It was not possible to look into the reproductive effects of these children as they were pre-purbertal but it was speculated leptin is also required to intitate puberty.
Farooqi et al (4) treated these children with human recombinant leptin daily for a year, weighing them daily, carrying out DEXA scans, recorded food intake and measured other hormone levels such as insulin, thyrotrophin and gonadotrophins. They lost on average 1-2kg per month, their appetite decreased and all other hormones were normal. Their GnRH became pulsatile after 12 months indicating leptin may affect puberty onset.
Another mouse with a similar phenotype to ob/ob mouse is the db/db mouse. It has very high leptin and was found to have a leptin receptor mutation due to a premature stop codon which codes for a receptor without the intraceullar C terminal, which is necessary for tyrosine kinase activation. Only one family have been found to have a leptin receptor mutation which were homozygotes were hyperphagic, obese, growth retarded and had hypothalamic hypothyroidism. This indicates that the lack of receptors causes certain hypothalamic releasing factors to be impaired. (1)
POMC
POMC (pre-pro-opiomelanocortin) is cleaved by prohormone convertases to form the melanocortin peptides adrenocorticotrophin (ACTH), melanocyte-stimulating hormones (MSH-alpha, beta and gamma) and beta-endorphin, an opiod receptor ligand. Discoveries about the link between alpha-MSH and regulation of food intake led to interest in the possible association of POMC with obesity. Scientists predicted, through knowledge of the normal physiology of POMC and melanocortin peptides, that the phenotype for POMC mutation would show obesity, pigmentation changes and ACTH deficiency. 2 patients showing this phenotype were initially found and sequencing of their POMC genes revealed mutations that abolished POMC function and therefore no melanocortin peptides could be detected in these patients. [10]
Many single nucleotide polymorphisms have now been identified that cause POMC deficiency syndrome, characterised as early-onset obesity, hyperphagia, hypocortisolaemia due to ACTH deficiency and often red hair with pale skin due to lack of ligand for melanocortin-1 receptors. However, pigmentation changes may be obscured by differing genetic backgrounds. Patients homozygous for POMC-null mutations exclusively exhibit the obesity phenotype but this is extremely rare. Heterozygous patients have been shown by analysis of family pedigrees to be more prone to obesity.[11]This has led to the suggestion that common obesity may be due to heterozygous genotypes for POMC mutations. In animal experiments, POMC-null heterozygous mice become obese on a high fat diet but remain slim on normal chow.[12] This indicates that environmental influences could affect whether heterozygous obesity genes result in the obesity phenotype. Extensive screenings of obese cohorts which don’t display other POMC-deficiency syndromic effects have not been conclusive about whether POMC mutations cause common obesity. In one study, only 6 out of 601 obese patients showed mutations in the non-coding regions of the POMC gene which could be linked to their obesity. [13]This suggests POMC mutations may not significantly contribute to common obesity but more research is needed to investigate this link.
MCR4
carboxypeptidase E
Polygenetic forms of Obesity
FTO
Conclusion
About References
To insert references and/or footnotes in an article, put the material you want in the reference or footnote between <ref> and </ref>, like this:
<ref>Person A ''et al.''(2010) The perfect reference for subpart 1 ''J Neuroendocrinol'' 36:36-52</ref> <ref>Author A, Author B (2009) Another perfect reference ''J Neuroendocrinol'' 25:262-9</ref>.
Look at the reference list below to see how this will look.[14] [15]
If there are more than two authors just put the first author followed by et al. (Person A at al. (2010) etc.)
Select your references carefully - make sure they are cited accurately, and pay attention to the precise formatting style of the references. Your references should be available on PubMed and so will have a PubMed number. (for example PMID: 17011504) Writing this without the colon, (i.e. just writing PMID 17011504) will automatically insert a link to the abstract on PubMed (see the reference to Johnsone et al. in the list.)
[16]
Use references sparingly; there's no need to reference every single point, and often a good review will cover several points. However sometimes you will need to use the same reference more than once.
How to write the same reference twice:
Reference: Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591
First time: <ref name=Berridge07>Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. ''Psychopharmacology'' 191:391–431 PMID 17072591 </ref>
Second time:<ref name=Berridge07/>
This will appear like this the first time [17] and like this the second time [17]
Figures and Diagrams
You can also insert diagrams or photographs (to Upload files Cz:Upload)). These must be your own original work - and you will therefore be the copyright holder; of course they may be based on or adapted from diagrams produced by others - in which case this must be declared clearly, and the source of the orinal idea must be cited. When you insert a figure or diagram into your article you will be asked to fill out a form in which you declare that you are the copyright holder and that you are willing to allow your work to be freely used by others - choose the "Release to the Public Domain" option when you come to that page of the form.
When you upload your file, give it a short descriptive name, like "Adipocyte.png". Then, if you type {{Image|Adipocyte.png|right|300px|}} in your article, the image will appear on the right hand side.
References
- ↑ WHO
- ↑ Neel JV. Diabetes mellitus: a 'thrifty' genotype rendered detrimental by 'progress'? Am J Hum Genetics 1962;4:352-3.
- ↑ McGarvey ST. Obesity in Samoans and a perpective on its etiology in Polynesians. Am J Clin Nutr 1991;53:1586S-94S
- ↑ Cournil A. Defay R. Lacroux A. Barny S. Fontbonne A. CALDIA Study Group. Paradoxical relationships between anthropometric variable and phenotypic expression of the metabolic syndrome in nondiabetic Polynesians of New Caledonia. Diabetes Care 2007;30(7):1909-11
- ↑ Ohashi J. et al. FTO polymorphisms in oceanic populations. J Hum Genet 2007;52:1031-1035
- ↑ Bouchard C. et al. Using MZ twins in experimental research to test for the presence of a genotype-environment interaction effect. Acta Genet. Med. Gemellol. 1990a; 39:85-89. PMID 2392894
- ↑ 7.0 7.1 Bouchard C.,Tremblay A. Genetic Influences on the Response of Body Fat and Fat Distribution to Positive and Negative Energy Balances in Human Identical Twins. Journal of Nutrition. 127; 5: 943S-947S PMID 9164270
- ↑ Hasselbalch AL et al. Associations between dietary intake and body fat independent of genetic and familial environmental background. Int J Obes (Lond). 2010 May;34(5):892-8 PMID 20125102
- ↑ Samaras K et al. Genes versus environment. The relationship between dietary fat and total and central abdominal fat. Diabetes Care. 1998 Dec;21(12):2069-76. PMID 9839096
- ↑ H Krude et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans 1998 Nature Genetics
- ↑ I. Sadaf Farooqi et al. Heterozygosity for a POMC-Null Mutation and Increased Obesity Risk in Humans Diabetes vol 55. Sept 2006
- ↑ B. G. Challis et al. Mice Lacking Pro-Opiomelanocortin Are Sensitive to High-Fat Feeding but Respond Normally to the Acute Anorectic Effects of Peptide- YY3-36. PNAS 2004
- ↑ H Krude et al. mutations in the proopiomelanocortin gene Annals of the New York Academy of Sciences June 2003
- ↑ Person A et al. (2010) The perfect reference for subpart 1 J Neuroendocrinol 36:36-52
- ↑ Author A, Author B (2009) Another perfect reference J Neuroendocrinol 25:262-9
- ↑ Johnstone LE et al. (2006)Neuronal activation in the hypothalamus and brainstem during feeding in rats Cell Metab 2006 4:313-21. PMID 17011504
- ↑ 17.0 17.1 Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591