Aromatic hydrocarbons: Difference between revisions
imported>Milton Beychok (Creating new stub article for Nathaniel Gunby to expand) |
imported>Nathaniel Gunby No edit summary |
||
| Line 1: | Line 1: | ||
{{subpages}} | <sub></sub>{{subpages}} | ||
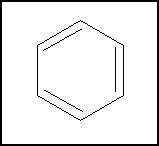
'''Aromatic hydrocarbons''' are | '''Aromatic hydrocarbons'''(aromatics) are a class of cyclic [[hydrocarbons]] with complete conjugation (which often means alternating double and single bonds). They tend to be more stable than normal alkenes. Not all fully conjugated cyclic hydrocarbons are aromatic, however- some act like normal alkenes, and one ([[cyclobutadiene]]) is anti-aromatic, and is even less stable than a normal alkene. The reasons for this are discussed below, under theoretical considerations. The most common aromatic hydrocarbon is [[benzene]], which was also the first to be discovered. Many larger aromatics consist of multiple benzene rings fused together. Benzene, alkyl benxenes (such as toluene) and fused-ring aromatics (such as anthracene) can all be isolated from coal tar. | ||
{{Image|Benzene DEVolk.jpg|right|350px|<small>Benzene, the simplest aromatic</small>}} | |||
==Reacticity== | |||
While aromatic hydrocarbons appear to contain mutliple double bonds, they undergo none of the reactions typical of [[alkene|alkenes]]. [[Catalyst|Catalytic]] addition of [[hydrogen]] to aromatics requires either higher pressures of hydrogen or more powerful catalysts than does addition to alkenes. [[Water]] will add to alkenes with [[acid]] catalysis, but will not add to aromatic rings. Some larger aromatic rings will undergo reactions more typical of alkenes. One of those that will is the middle ring of anthracene. | |||
In general,[[electrophile|electrophiles]] replace a hydrogen atom of an aromatic ring instead of adding to the double bonds. The most common reactions include: | |||
;'''Nitration''': A mixture of [[nitric acid|nitric]] and [[sulfuric acid|sulfuric]] acids is used to replace a hydrogen atom with a [[nitro]] (NO<sub>2</sub>) group. This can be reduced to an [[amine]] if desired. | |||
;'''Sulfonation''': Oleum, a mixture of very contentrated sulfuric acid and sulfur trioxide, can be used to replace a hydrogen with a SO<sub>3</sub>H group. These [[sulfonic acids]] are used to make [[sulfonamide|sulfa drugs]], and are also strong acids soluble in organic solvents (unlike some of the strong mineral acids) | |||
;'''Friedel-Crafts acylation''': This process uses a carboxylic acid chloride and AlCl<sub>3</sub> to introduce an acyl group, which forms a benzyl [[ketone]]. This can be reduced to an alkylbenzene if desired. | |||
;Friedel-Crafts alkylation:This process, which uses an alkyl halide and AlCl<sub>3</sub> catalyst to introduce an alkyl group, was discovered before the acylation reaction (see above). However, the alkylation is less reliable, so acylation is often used instead. | |||
;Halogenation:This uses Br<sub>2</sub> or Cl<sub>2</sub>, along with AlCl<sub>3</sub> or FeBr<sub>3</sub> as a catalyst, to introduce a [[bromine]] or [[chlorine]] atom. | |||
==Theoretical Considerations== | |||
Revision as of 16:02, 2 November 2010
Aromatic hydrocarbons(aromatics) are a class of cyclic hydrocarbons with complete conjugation (which often means alternating double and single bonds). They tend to be more stable than normal alkenes. Not all fully conjugated cyclic hydrocarbons are aromatic, however- some act like normal alkenes, and one (cyclobutadiene) is anti-aromatic, and is even less stable than a normal alkene. The reasons for this are discussed below, under theoretical considerations. The most common aromatic hydrocarbon is benzene, which was also the first to be discovered. Many larger aromatics consist of multiple benzene rings fused together. Benzene, alkyl benxenes (such as toluene) and fused-ring aromatics (such as anthracene) can all be isolated from coal tar.
Reacticity
While aromatic hydrocarbons appear to contain mutliple double bonds, they undergo none of the reactions typical of alkenes. Catalytic addition of hydrogen to aromatics requires either higher pressures of hydrogen or more powerful catalysts than does addition to alkenes. Water will add to alkenes with acid catalysis, but will not add to aromatic rings. Some larger aromatic rings will undergo reactions more typical of alkenes. One of those that will is the middle ring of anthracene.
In general,electrophiles replace a hydrogen atom of an aromatic ring instead of adding to the double bonds. The most common reactions include:
- Nitration
- A mixture of nitric and sulfuric acids is used to replace a hydrogen atom with a nitro (NO2) group. This can be reduced to an amine if desired.
- Sulfonation
- Oleum, a mixture of very contentrated sulfuric acid and sulfur trioxide, can be used to replace a hydrogen with a SO3H group. These sulfonic acids are used to make sulfa drugs, and are also strong acids soluble in organic solvents (unlike some of the strong mineral acids)
- Friedel-Crafts acylation
- This process uses a carboxylic acid chloride and AlCl3 to introduce an acyl group, which forms a benzyl ketone. This can be reduced to an alkylbenzene if desired.
- Friedel-Crafts alkylation
- This process, which uses an alkyl halide and AlCl3 catalyst to introduce an alkyl group, was discovered before the acylation reaction (see above). However, the alkylation is less reliable, so acylation is often used instead.
- Halogenation
- This uses Br2 or Cl2, along with AlCl3 or FeBr3 as a catalyst, to introduce a bromine or chlorine atom.