Filtration: Difference between revisions
imported>Madeline Schiesser m (→Filter aid) |
imported>Madeline Schiesser m (→Filter aid) |
||
| Line 44: | Line 44: | ||
===Filter aid=== | ===Filter aid=== | ||
{{Image|IMG 1925.JPG|right| | {{Image|IMG 1925.JPG|right|300px|Two jars of diatomite filter aid with dishes of the filter aid powder in the foreground.}} | ||
Filtration can be improved by adding filter aid, a kind of highly permeable powdered solid, to the feed side of the filter medium. When choosing a filter aid for a process, factors such as surface properties, chemical resistance, and compatibility with the product should be considered. | Filtration can be improved by adding filter aid, a kind of highly permeable powdered solid, to the feed side of the filter medium. When choosing a filter aid for a process, factors such as surface properties, chemical resistance, and compatibility with the product should be considered. | ||
Revision as of 19:06, 20 December 2010
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 21 December 2010. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! Note to course participants: Looking forward to some insightful and useful articles from your collaborations. |
This article is about Conventional Filtration, for crossflow filtration see Crossflow filtration.
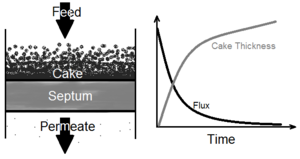
Filtration is a process by which most or all solid particles in a liquid-solid suspension fluid are separated from the fluid by passing the fluid through a porous medium barrier. In conventional filtration (as opposed to crossflow filtration in which flow is parallel) the incoming fluid, or feed, flows perpendicular to the porous medium. A layer of solids builds up over time on the surface of the medium, while the flux of the feed through the medium approaches a zero asymptote. In bioprocessing, filtration is an early processing step during the recovery phase. Bioprocessing filtration may be employed, for example, to retrieve a target product in solution from an undesired solid mass of freshly ruptured cells. Filtration is particularly valuable in early processing because it allows for an efficient means of volume reduction. Filtration, dating back in its simplest form to water treatment around 2000 B.C.E., is well established in the fields of science and engineering, having seen little to no change in established theory in the past century, although innovative advances have been made in this century in the quality and efficiency of equipment associated with the filtration process.
The Processes
Filtration deals with the separation of solids in suspension from liquids. A barrier, called the filter medium or septum, catches solid particles that are too large to pass through this medium on an upstream side, while liquids and very fine solid particles pass through the medium to a downstream side. On the septum's upstream surface, the larger solids, caught by the filter medium, form a continuously thickening layer known as a filter cake. As this cake increases in thickness, the flux of material, or flow of filtrate through the filter medium, decreases, approaching a horizontal asymptote at zero.[1] It is therefore necessary to occasionally stop filtration and remove the cake, or systematically clean the filter during filtration, in order to continue a process of filtration for an extended period of time. Several methods and devices have been devised that allow for this filter cleaning while operating, as well as mechanisms permitting easy removal of filtered solids from the filtering machinery.
In mathematical modeling, filter cakes are often treated as non-compressible. Unfortunately, that is not the case in practice. Several factors, including the pressure gradient of the driving force, if applicable, may cause the filter cake to partially collapse. This can make determining the height and resistance of the cake difficult, which in turn renders other variables such as flux and processing time indeterminate.
Design and Operation
There are four categories of filtration as divided by driving force: gravity, vacuum, pressure and centrifugal force. The last may also be considered a form of centrifugation, and will not be discussed in great detail here. Types of filtration may also be classified by mechanism (sorted by whether solids are stopped at the surface of a cake or trapped within pores of a medium), by objective (sorted by whether a dry solid, clarified liquid, or both are sought), by operating cycle (sorted by whether filtration is continuous or intermittent), or by nature of the solids.[2] For simplification, only classification by driving force, with specific reference to exemplary machinery will be discussed here.
Gravity filtration
The simplest form of filtration uses only the force of gravity to drive a fluid through a porous medium. Since this driving force is relatively weak, the filtrate must be of low viscosity and the slurry generally dilute. The medium is placed orthogonal to the force of gravity and parallel to the ground. An excellent example of such filtration may be found in the common place household drip coffee filter, in which a layer of coffee grounds acts as a pre-established filter cake and the thin paper of the coffee filter is the filter septum. Liquid coffee streams through the septum into a collecting pot below; coffee grounds remain trapped on the upper side of the septum. Such gravity filters may also be used in industry on a larger scale, for example, the sand gravity filter used in drinking water treatment, however, with only gravity as the driving force, these filters tend to be too slow to meet the demands of most industries. [3]
Vacuum filtration
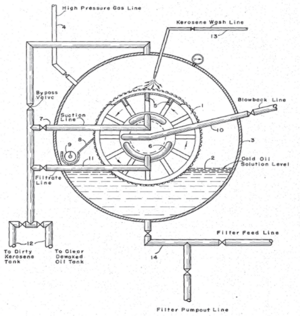
A vacuum filter operates by creating a lower pressure on the opposite side of the porous medium with respect to the incoming feed. A vacuum may provide a substantial pressure difference sufficient to filter a filtrate too viscous for a gravity filter to handle. A vacuum filter can also produce a fairly dry solid and provide a means for washing the precipitate. Examples of vacuum filters include the leaf filter, the Buchner funnel, and rotating drum vacuum filters.
Leaf filters are a staple of this design. Essentially, the leaf filters with a low internal pressure (due to vacuum) are immersed in the filtrate mixture until a thick layer of cake has built up on the surface of the leaves. The leaves are then transferred to a washing tank, where wash water is drawn through each leaf until an internal pressure is applied to the leaf, removing the cake. In another example, drums with filter media on the outer face, and an internal vacuum, are partially immersed in and rotated through the filtrate mixture, picking up a layer of solids. As the drum rotates out of the mixture, the precipitate is washed off the surface and collected. Most vacuum filters operate continuously.[3]
Pressure filtration
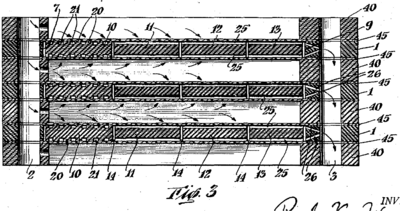
If a higher pressure is required than can be achieved via vacuum filtration, such as when an especially viscous liquid is being filtered or filter-aid cannot be used to ease the filtration (see below), a pressure filter may be employed. In pressure filtration, the filtrate is driven through the filter by positive pressure on the feed side of the filter. While there are several forms of pressure filters, the filter press largely dominates the field. Often taking the form of the plate-and-frame filter or the similar plate filter, the filter press is comprised of a series of plates and frames between which the filtering medium is arranged such that the plates tightly stack one against the next, forming small chambers in between. Channels for filtrate or wash and clarified fluid or soiled wash run along the top and bottom of the plate stack. Near the top and bottom of each plate are holes which may alternately be shut to force filtrate or wash entering one hole to flow through the medium to exit an alternate hole on the side of the filter distal to the side from which the filtrate entered. After filtrate has run through the filter press for a time, cake builds up on one side of the filters. The holes may alternately be plugged and wash forced through the device to clean the cake from the filter press device. Pressure filtration has high energy demands and the device cleaning and cake removal process is often sloppy and messy, however, this form of filtration is capable of separating solid-liquid suspensions that other forms of filtration are simply not capable of addressing.[3]
Centrifugal force filtration
Traditional centrifuge devices, such as the basket centrifuge, may be adapted to act as a filter by placing a filter medium orthogonal to the centripetal force. Solids are caught by the medium and may generally be removed by the same arrangement used to remove solids from the traditional centrifuge, such as by stopping the centrifuge or by continuous removal from a rotating element.[3]
Materials
Under Construction
Filter media
Will discuss various materials used as filter medium, when they are used, pros/cons as applicable.[4]
Filter aid
Filtration can be improved by adding filter aid, a kind of highly permeable powdered solid, to the feed side of the filter medium. When choosing a filter aid for a process, factors such as surface properties, chemical resistance, and compatibility with the product should be considered.
The filter aid may be mixed directly with the filtrate or a layer of filter aid may be applied direcly to the medium. The objective of the former approach is to keep the pores in the filter cake open, to prevent compression of the filter cake, and to hasten the speed of filtration. In some instances of the latter approach, such as rotary vacuum filter, the filter is operated with a layer of filter aid "precoat." When operating with a precoat, the filter medium is coated with a thick layer of filter aid and the solids being filtered penetrate into this layer of precoat a small distance. An adjustable knife is used to cut away the layer of precoat containing filtered solids, exposing a fresh layer of precoat. When the layer of precoat on the medium becomes very thin, a new, thick layer of precoat is applied as stated above and the process repeats.
Diatomite (kieselguhr or deatomaceous earth) and perlite are two of the prevalent filter aids in use. Diatomite is made from the skeletal remains of diatoms, a kind of algae whose structure provides for high intrinsic permeability and whose chemical makeup of mostly silica allows for insolubility in both strong acids and alkalies. Perlite is a glassy volcanic material, having a high aluminum silicate content, whose high porosity comes from its ability to expand on heating during processing. Perlite is popular for rough filtrations where filtrate clarity is of low priority by high flow rates are preferred.
Principles and theory
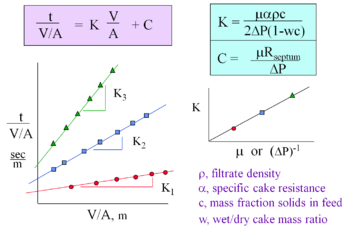
In most cases of conventional filtration, a solid suspension fluid, or filtrate, flows against a porous medium by application of a pressure gradient across the medium, where the solids in the suspension too large to pass through the medium become trapped on one side of the medium, building up in a layer called a cake, or filter cake. The flow of the liquid filtrate through the porous medium, which is a bed of solids, may be described by Darcy's Law written in the form:
where A (m2) is the cross-sectional area of the medium through which the fluid flows, V (m3) is the volume of filtrate, t (sec) is the time, Δp (Pa) is the change in pressures across the medium and cake, μo (kg/m*s) is the is the viscosity of the filtrate, and R (m-1) is the combined (in series) resistance of the filter medium ("RM") and filter cake ("RC"), which is:
- .
The cake resistance may further be described in terms of the specific cake resistance, α (m/kg),in the following form:
where ρc (kg/m3) is the mass of dry cake solids per unit volume of filtrate.
Darcy's Law for the flow of the liquid filtrate through the bed of solids porous filter medium and cake may therefore be rewritten as:
For constant pressure operation, this equation may be integrated with an initial condition of zero filtrate at time zero to yield:
which may also be rewritten in term of the filtrate density ρ (kg/m3), the ratio of the mass of dry cake solids to the mass of feed c (kg/kg), and the ratio of the mass of wet cake to the mass of dry cake w (kg/kg):
A method of graphing may be used, as seen on the right, to find and . The integrated Darcy's Law equation above is graphed to find the slope equaling K and the y-intercept which is C.[1]
History
Filtration is well established in the arts of science and engineering. Simple methods of filtration were used by ancient civilizations, and are recorded in both Sanskrit writings and Egyptian inscriptions. As early as 2000 B.C.E. filtration through sand, coarse gravel, and charcoal were known methods of treating drinking water. In Greece, Hippocrates (460–354 B.C.E.) advised straining rain water after boiling through a cloth bag, called a “Hippocrates’ sleeve,” to prevent the water from smelling foul and causing hoarseness.[5] In the ancient era, wine-makers also used cloth-covered screens to removed unwanted impurities from wine.
The first modern demonstration of sand filtration was in a 17th century text by Italian physician Lucas Antonius Portius in which he described three pairs of sand filters, each having a downward-flow and an upward flow filter through which water flowed. In approximately 1703, La Hire, a Parisian scientist, brought a plan before the French Academy of Sciences in which he proposed the widespread use of rainwater cisterns and sand filters in every household to promote the use and availability of clean water. It would take another century until his vision was practiced.[5]
In 1804, Paisley, Scotland saw the first municipal water treatment plant consisting of a sand and gravel filter whose water was distributed by horse and cart. Shortly thereafter, Glasgow, Scotland began distributing water to its residents using a system of pipes. Robert Thom designed a slow sand filter, cleaned using backwash, which was put into use in Greenock, Scotland by 1827. James Simpson designed a similar filter, cleaned by scraping, which was used in London by 1829 and became the English standard around the world. Slow sand filters were the chosen method for cleaning municipal water supply in the 19th century, however, these filters were problematically large in size, with sand beds 2 to 3 feet (0.5 to 1 meter) deep, areas covering several acres, and cleaning methods requiring scrapping with a shovel and brute effort. Given the rate at which cities were expanding and their increasing need for land, the slow sand filter could not produce a sufficient volume of water to justify its enormous size. In the 1880s, rapid sand filtration was developed in the United States by improving upon Thom's methods. The false bottom and reverse-flow wash of the slow sand filter was kept, but the rapid sand filter also used water jets or backwash to clean the filter medium and a mechanical agitator to loosen debris. Pretreatments (e.g. coagulation and settling) also became increasingly popular as a means to decrease the amount of actual sediment the filter would need to process and thereby effectively decrease the size requirements of the sand filters. Eventually, filtration through charcoal was used to increase the taste and odor of water, although this method was initially thought to be unfeasible for large municipal water supplies.[5]
The theory behind filtration is well established and has been for well over a century, and through mankind's trails with water purification via filtration, the use of filtration for separating other liquids and solids has been easily extrapolated. In the past century there have not been any major changes in filtration theory, as such is already well understood. In addition to gravity, as primarily discussed above, pressure and centripetal force were also employed to drive filtrate through filter media as the technology to harness these forces was developed. Small advances and innovations have been made in filtration equipment in the past century to increase the efficiency and quality of filtration. However, the basic principles remain unchanged. A comparison of chemical engineering texts from 1937, 1952, 1997, and 2003, all revealed the same understanding of theory and point to very similar illustrative devices to demonstrate example filters.
References
- ↑ Roger G Harrison, Paul Todd, Scott R. Rudge, and Demetri P. Petrides, "Filtration," Bioseparations Science and Engineering. New York: Oxford U P, 2003.
- ↑ Robert H. Perry and Don W. Green, "Filtration," Perry's Chemical Engineering Handbook. New York: McGraw-Hill, 1997.
- ↑ B. E. Lauer and Russell F. Heckmam, "Separation of a Solid from a Liquid," Chemical Engineering Techniques. New York: Reinhold, 1952.
- ↑ William H. Walker, Warren K. Lewis, William H. McAdams and Edwin R. Gilliland, "Filtration," Principles of Chemical Engineering. New York: McGraw-Hill, 1937.
- ↑ Kathy Jesperson. "Search for Clean Water Continues," On Tap. National Environmental Services Center, West Virginia University. Summer 1996. 18 December 2010 <http://www.nesc.wvu.edu/old_website/ndwc/ndwc_DWH_1.html>