Natural gas: Difference between revisions
John Leach (talk | contribs) m (Text replacement - "hydrogen sulfide" to "hydrogen sulphide") |
mNo edit summary |
||
| Line 182: | Line 182: | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:01, 24 September 2024
Natural gas is a gas consisting primarily of methane (CH4). It is found as raw natural gas in underground reservoirs, as gas associated with underground reservoirs of petroleum crude oil, as undersea methane hydrates and as coalbed methane in underground coal mines. It is an important fuel source and a major feedstock for producing ammonia, hydrogen, petrochemicals and fertilizers.
Natural gas is often informally referred to as simply gas or fuel gas, especially when compared to other energy sources such as electricity.
There are a great many different natural gas reservoirs worldwide and each of those gas deposits has a different composition. However, in general, most of them contain at least 80 to 90 volume percent of methane. Before raw natural gas extracted from those reservoirs can be used as a fuel, it must undergo extensive processing to remove almost all materials other than methane. The by-products of that processing include ethane, propane, butanes, natural gasoline consisting of pentanes plus higher molecular weight hydrocarbons (often referred to as pentanes+ ), elemental sulphur, and sometimes helium and nitrogen.
Formation of natural gas
While the origins of natural gas deposits are not known with certainty, and the different reservoirs vary in their geologic history, it is generally believed[1][2] that the methane-rich gas was formed over millions of years from organic matter, usually from former marine or coastal ecosystems. Decomposed organic matter from dead plants and animals became covered in layers of mud and other sediments, and as more mud and other sediments piled on top of the organic matter, the pressure and temperature increased. The increased pressure and temperature is thought to have caused the organic matter to slowly transform into methane-rich gas and oil. Once formed, gas deposits tended to rise towards the surface of the earth through fissures and pores, until they become trapped underneath less permeable rock layers and formed gas reservoirs.
Composition of raw natural gas
Raw natural gas typically consists primarily of methane (CH4), the shortest and lightest hydrocarbon molecule. It also contains varying amounts of:
- Higher molecular weight gaseous hydrocarbons: Raw natural gas may contain a total of as much as 20 volume per cent of ethane (C2H6), propane (C3H8), normal butane (n-C4H10), isobutane (i-C4H10), pentanes and even higher molecular weight hydrocarbons. When processed and purified into finished by-products, all of these are collectively referred to as NGL (Natural Gas Liquids).
- Acid gases: carbon dioxide (CO2), hydrogen sulphide (H2S) and mercaptans such as methanethiol (CH3SH) and ethanethiol (C2H5SH).
- Other gases: nitrogen (N2) and helium (He).
- Water: water vapor and liquid water.
- Liquid hydrocarbons: perhaps some natural gas condensate, a low-boiling point mixture of liquid hydrocarbons called natural gas condensate, sometimes also called natural gasoline, casinghead gasoline or simply condensate.
- Mercury: very small amounts of mercury primarily in elemental form, but chlorides and other species are possibly present.[3]
Measurement units and heating values
Quantities of natural gas are measured in normal cubic meters at 0 °C and 101.325 kPa absolute pressure or in standard cubic feet at 60 °F and 14.496 psi absolute pressure.[4]
The higher heating value (HHV)[5] of commercial, processed natural gas is about 40 MJ per normal cubic meter which, in the United States, is equivalent to about 1,015 Btu per standard cubic foot. However, those values can vary by several percent from one natural gas to another depending upon their source reservoir and upon their degree of processing.
The heating value of a fuel gas when the water formed during combustion does not condense is the called the lower heating value (LHV)[6] and can be as much as 10% less than the HHV.
In the United States, retail sales of processed natural gas to end users are often measured in units of therms with 1 therm being 100,000 Btu (equivalent to the higher heating value of roughly 100 standard cubic feet).
Finding and extracting natural gas
- Exploration
Exploration, in the oil and gas industry, is the search for underground or undersea natural gas reservoirs. Recoverable reserves of natural gas tend to occur where impermeable rocks (called caprocks) constrain the upward movement of gas through the underground soil and rocks. Geologists and geophysicists usually use seismology to find areas which have the right conditions for a gas or oil deposit. High-speed computers that help develop three-dimensional underground maps as well as satellite image technology are also widely used to search for natural gas reservoirs. The various worldwide areas having significantly large reservoirs of natural gas are referred to as gas fields and most gas fields have been given a unique name, such as the North Field in Qatar. Many gas fields are in underwater gas reservoirs and are referred to as offshore fields, such as in the North Sea, the Gulf of Mexico, the Persian Gulf and elsewhere.
- Extraction
After an initial exploration, wells are drilled to confirm the existence of a reservoir and its size. Once a reservoir of natural gas has been confirmed, production wells are drilled to extract the gas (see adjacent photograph). Once reached by a well, the gas comes up to the surface under its own pressure.
Over time, the gas flow rate decreases, but may be maintained by drilling reinjection wells and using gas compressors to reinject some compressed gas downward into the reservoir.
Also over time, as gas is extracted, the pressure in the underground gas reservoir declines. When it decreases to below its hydrocarbon dew point, any propane, butanes and pentanes and higher molecular weight hydrocarbons that it contains will partially condense into liquids. That formation of liquid hydrocarbons in a gas reservoir is called retrograde condensation because some of the gas condenses into a liquid under isothermal conditions instead of expanding or vaporizing when the pressure is decreased. The liquids thus formed may get trapped by depositing in the pores of the gas reservoir. One method of mitigating this problem is to reinject dried gas, free of condensate, to maintain the underground pressure and to allow re-evaporation and extraction of the condensate.
The upper adjacent photograph depicts the extent of a gas field and the many wells drilled to extract natural gas from the underground gas reservoir. The lower photograph depicts a typical gas drilling rig.
Sources of natural gas
| ||||||||||||||||||||||||||||
Types of natural gas sources
Raw natural gas is presently extracted primarily from any one of three conventional underground sources, namely petroleum crude oil wells, gas wells, and condensate wells:
- Raw natural gas that comes from petroleum crude oil wells is typically termed associated gas. This gas can exist separate from the crude oil in the underground formation, or dissolved in the crude oil.
- Raw natural gas from gas wells and from condensate wells contains little or no crude oil and is termed non-associated gas. Gas wells typically produce only raw natural gas, while condensate wells produce raw natural gas along with natural gas condensate.
Methane-rich gas can also come from methane deposits in the pores of some coal seams. Such gas is referred to as coalbed gas or coalbed methane (CBM) and it is also called sweet gas because it is relatively free of hydrogen sulfide. It consists mainly of methane with only trace quantities of ethane, nitrogen and carbon dioxide. It does not contain propane, butanes, pentanes or condensate.
Natural gas hydrate (also called clathrate hydrate, methane clathrate or methane hydrate) is a solid in which methane is trapped within the crystal structure of ice. Significant deposits of methane clathrate have been found under sediments on Earth's ocean floors and in the arctic regions.[9] The amount of the worldwide reserves of methane clathrates is poorly known, and estimates of those reserves have decreased many orders of magnitude[10] since it was first recognized that methane clathrates could exist in the oceans during the 1960s. Recent estimates (about 2003) range from 1 to 5 × 1015 m3 of methane.[11] That amounts to 6 to 29 times the worldwide proven natural gas reserves of 175.4 × 1012 m3 from conventional sources as shown in Table 1. As of 2009, there is no available technology for economically extracting and exploiting methane from the hydrate reservoirs.
Biogas, landfill gas, town Gas and synthetic gas (produced by coal gasification) are man-made fuels. They are not natural gases.
Natural gas reserves
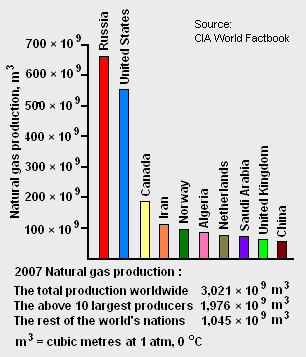
The world's total proven reserves[7] of natural gas as of 2007 amounted to 175.4 × 1012 cubic metres and the country having the largest proven reserves was Russia with 44.7 × 1012 cubic metres (see Table 1). As shown in Figure 1, Russia was also the world's largest natural gas producer in 2007.
The world's largest gas field is the offshore North Field in Qatar, estimated to have 25 × 109 cubic metres of gas in place. The second largest natural gas field is the South Pars Gas Field in Iranian waters in the Persian Gulf. Connected to Qatar's North Field, it has estimated reserves of 8 to 14 × 109 cubic metres.[12]
Natural gas processing
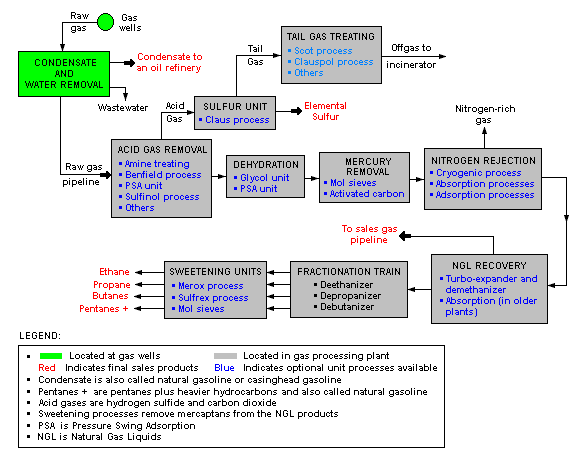
There are a great many ways in which to configure the various unit processes used in the processing of raw natural gas. The schematic block flow diagram below is a generalized, typical configuration for the processing of raw natural gas from non-associated gas wells. It shows how raw natural gas is processed into sales gas pipelined to the end user markets.[13][14][15]
The block flow diagram also shows how processing of the raw natural gas yields these byproducts:
- Natural gas condensate
- Sulfur
- Ethane
- Natural gas liquids (NGL): propane, butanes and C5+ (which is the commonly used term for pentanes plus higher molecular weight hydrocarbons)
Transportation and storage
The major means of transporting large volumes of natural gas overland is by pipelines and pipelines have been established as the preferred transportation mode for overland distances of up to about 4,000 kilometres (2,500 miles).
However, transporting significant amounts of natural gas across oceans in pipelines is too expensive and using ordinary seagoing tankers is not feasible because of the very large volume of a gas compared to its liquid volume. However, natural gas can be and has been cryogenically cooled to – 161 °C (– 258 °F), condensed into liquified natural gas (LNG) and transported in large LNG tankers capable of carrying about 150,000 m3 of LNG at that temperature and at essentially atmospheric pressure. Transforming natural gas to a liquid is accompanied by a volume reduction of approximately 600 to 1. Thus, each large tanker can transport LNG which can be reconverted to 90,000,000 m3 of natural gas at atmospheric pressure when delivered at the end of its journey.
For LNG transportation by seagoing tankers, an LNG liquefaction plant is needed at the exporting end and an LNG regasification plant is needed at the receiving LNG terminal. LNG transportation by seagoing LNG tankers has been established as the preferred technology for long distance transportation across oceans.
LNG carriers can be used to transport liquefied natural gas (LNG) across oceans, while tank trucks can carry liquefied or compressed natural gas (CNG) over shorter distances. Sea transport using CNG carrier ships that are now under development may be competitive with LNG transport in specific conditions.
Compressed natural gas (CNG) is a substitute fuel for the use of gasoline, diesel fuel, or propane in automotive vehicles. CNG is natural gas that has been compressed to an absolute pressure of about 200 to 220 bar (2,900 to 3,190 psi) which results in reducing the gas volume to about 0.5% of its volume at atmospheric pressure. CNG is transported by rail or in tanker trucks overland to end-users or to distribution points such as pipelines for further transport. As of 2009, plans and designs have been developed for using seagoing CNG tankers for oversea transport in the near future and such transport is expected to be competitive to LNG oversea transport in LNG tankers.
Natural gas is often stored underground inside depleted gas reservoirs from previous gas wells, salt domes, or in storage tanks as liquefied natural gas. The gas is stored during periods of low demand and extracted during periods of higher demand.
Uses of natural gas
- Power generation
Natural gas is a major source of electricity generation through the use of gas turbines and steam turbines. Most grid peaking power plants and some off-grid engine-generators use natural gas. Particularly high efficiencies can be achieved through combining gas turbines with a steam turbine in combined cycle mode. Natural gas burns more cleanly than other fossil fuels, such as oil and coal, and produces less carbon dioxide per unit energy released. For an equivalent amount of heat, burning natural gas produces about 30% less carbon dioxide than burning fuel oil and about 45% less than burning coal.[16] Combined cycle power generation using natural gas is thus the cleanest source of power available using fossil fuels, and this technology is widely used wherever gas can be obtained at a reasonable cost. Fuel cell technology may eventually provide cleaner options for converting natural gas into electricity, but as yet it is not price-competitive.
- Domestic use
Natural gas is supplied to homes, where it is used for such purposes as cooking in natural gas-powered ranges and/or ovens, natural gas-heated clothes dryers, heating/cooling and central heating. Home or other building heating may include boilers, furnaces, and water heaters. Compressed natural gas (CNG) is used in rural homes without connections to piped-in public utility services, or with portable grills. However, due to CNG being less economical than LPG which is liquified propane, butane or a mixture of both, LPG is the dominant source of rural gas.
- Transportation fuel
Compressed natural gas (CNG) is a cleaner alternative to other automobile fuels such as gasoline (petrol) and diesel fuel. As of December 2008, the countries with the highest number of CNG vehicles, ranked numerically, were Pakistan [17], Argentina, Brazil, Iran and India. The energy efficiency is generally equal to that of gasoline engines, but lower compared with modern diesel engines. Gasoline/petrol vehicles converted to run on natural gas suffer because of the low compression ratio of their engines, resulting in a cropping of delivered power while running on natural gas (10%-15%). CNG-specific engines, however, use a higher compression ratio due to this fuel's higher octane number of 120–130.[18]
- Fertilizer
Natural gas is a major feedstock for the production of ammonia used in fertilizer production.
- Hydrogen
Natural gas is also a major feedstock for the production of hydrogen, with one common method being the steam-methane reforming (SMR) process.. Hydrogen has various applications: it is a primary feedstock for the chemical industry, a hydrogenating agent, an important commodity for oil refineries, and a fuel source in hydrogen vehicles.
- Aviation
Russian aircraft manufacturer Tupolev is currently running a development program to produce LNG- and hydrogen-powered aircraft.[19] The program has been running since the mid-1970s, and seeks to develop LNG and hydrogen variants of the Tu-204 and Tu-334 passenger aircraft, and also the Tu-330 cargo aircraft.
The advantages of liquid methane as a jet engine fuel are that it has more specific energy than the standard kerosene mixes and that its low temperature can help cool the air which the engine compresses for greater volumetric efficiency, in effect replacing an intercooler. Alternatively, it can be used to lower the temperature of the exhaust.
- Other
Natural gas is used as a feedstock in the production of petrochemicals.
Environmental effects
The use of natural gas as combustion fuel produces far less emissions of air pollutants such as sulfur dioxide and nitrogen oxides than does the combustion of coal or fuel oils. However, it does contribute significantly to global carbon dioxide emissions. According to the IPCC Fourth Assessment Report, in 2004 natural gas produced about 5,300 Mt/year of carbon dioxide emissions, while coal and oil produced 10,600 and 10,200 Mt/yr respectively.
In addition, any methane-rich gas such as natural gas is a greenhouse gas more potent than carbon dioxide when released into the atmosphere. It is inevitable that, in using natural gas as a combustion fuel or a petrochemical feedstock on a large scale, some of it will leak into the atmosphere. However, it is not of large concern due to the small amounts of such leakage. Carbon dioxide receives the most attention over greenhouse gases because it is in much higher concentrations.
Extraction of natural gas leads to a decrease of the pressure in an underground gas reservoir. This in turn may sometimes lead to subsidence at ground level. Subsidence can affect ecosystems and waterways as well as sewer and water supply systems. Large gas fields degrade the aesthetics of rural or wilderness areas.
Safety
Processed natural gas is colorless and essentially odorless. Since it is also very flammable, it is important that natural gas leaks be detected before a fire or explosion occurs. For that reason, very small amounts of a mercaptan such as t-butyl mercaptan, with a strongly unpleasant smell, is added to the natural gas as an odorant. Such odorants are considered non-toxic in the extremely low concentrations at which they are used. Odorizing of natural gas began in the United States after the 1937 New London School explosion in the town of New London, Texas.[20] The buildup of gas in the school's basement went unnoticed, killing three hundred students and faculty when it ignited.
In coal mines, where any coalbed methane that may be present has no odor, methane sensors are used, and mining equipment has been specifically developed to avoid ignition sources.
Explosions caused by natural gas leaks occur a few times each year. Individual homes, small businesses and boats are most frequently affected when an internal leak builds up gas inside the structure. Frequently, the blast will be enough to significantly damage a building but leave it standing. In such cases, the people inside tend to have minor to moderate injuries. Occasionally, the gas can collect in high enough quantities to cause a deadly explosion, disintegrating one or more buildings in the process. The gas usually dissipates readily outdoors, but can sometimes collect in dangerous quantities if weather conditions are right. However, considering the tens of millions of structures that use the fuel, the individual risk of using natural gas is very low.
Natural gas heating systems are a minor source of carbon monoxide deaths in the United States. According to the US Consumer Product Safety Commission,[21] 56% of unintentional deaths from non-fire carbon monoxide poisoning were associated with engine-driven tools like gas-powered generators and lawn mowers. Natural gas heating systems accounted for 4% of these deaths. Improvements in natural gas furnace designs have greatly reduced CO poisoning concerns. Detectors are also available that warn of unsafe levels of carbon monoxide and/or explosive gas (methane, propane, etc.).
References
- ↑ Natural Gas Formation, Exploration, and Distribution From the website of the Pacific Gas and Electricity Company.
- ↑ Natural Gas From the website of the Climate Lab
- ↑ Mercury Removal from Natural Gas and Liquids UOP website page
- ↑ Air Dispersion Modeling Conversions and Formulas 1 normal cubic meter at 0 °C and 101.325 kPa absolute = 37.326 standard cubic feet at 60 °F and 14.695 psi absolute.
- ↑ Also referred to as the gross heating value (GHV)
- ↑ Also referred to as the net heating value (NHV)
- ↑ 7.0 7.1 Proved reserves are those quantities of natural gas, which, by analysis of geological and engineering data, can be estimated with a high degree of confidence to be commercially recoverable from a given date forward, from known reservoirs and under current economic conditions.
- ↑ CIA World Factbook Country Comparison :: Natural gas - proved reserves
- ↑ A Global Inventory of Natural Gas Occurrence As of April, 2008. From the website of the U.S. Geological Survey
- ↑ Orders of magnitude are powers of 10. One order of magnitude is factor of 10, two orders of magnitude is a factor of 100, three orders of magnitude is a factor of 1,000, etc.
- ↑ A.V. Milkov (2004). "Global estimates of hydrate-bound gas in marine sediments: how much is really out there?". Earth-Sci Rev 66 (3-4): 183–197. DOI:10.1016/j.earscirev.2003.11.002. Research Blogging.
- ↑ Pars Special Economic Energy Zone. Pars Special Economic Energy Zone. Retrieved on 2007-07-17.
- ↑ Natural Gas Processing: The Crucial Link Between Natural Gas Production and Its Transportation to Market
- ↑ Example Gas Plant
- ↑ NaturalGas.org - Processing Natural Gas
- ↑ Natural Gas and the Environment
- ↑ Natural Gas Vehicle Statistics. International Association for Natural Gas Vehicles (2008-12-31). Retrieved on 2009-06-11.
- ↑ Clean Engine Vehicle, Measurement and Control Laboratory
- ↑ Flightglobal: Tupolev's cryogenic airliners
- ↑ New London School Explosion
- ↑ Incidents, Deaths, and In-Depth Investigations Associated with Non-Fire Carbon Monoxide from Engine-Driven Generators and Other Engine-Driven Tools
- Editable Main Articles with Citable Versions
- CZ Live
- Engineering Workgroup
- Chemistry Workgroup
- Chemical Engineering Subgroup
- Energy policy Subgroup
- Articles written in American English
- Advanced Articles written in American English
- All Content
- Engineering Content
- Chemistry Content
- Chemical Engineering tag
- Energy policy tag