Post irradiation examination: Difference between revisions
imported>Mark Rust |
imported>Mark Rust |
||
| Line 206: | Line 206: | ||
According to the report on Chernobyl used in the above table 3.5% of the following isotopes in the core were released <sup>239</sup>Np, <sup>238</sup>Pu, <sup>239</sup>Pu , <sup>240</sup>Pu, <sup>241</sup>Pu and <sup>242</sup>Cm. | According to the report on Chernobyl used in the above table 3.5% of the following isotopes in the core were released <sup>239</sup>Np, <sup>238</sup>Pu, <sup>239</sup>Pu , <sup>240</sup>Pu, <sup>241</sup>Pu and <sup>242</sup>Cm. | ||
==Contact of molten fuel with water and concrete== | |||
While much of this work is strictly PIE it is important to understand the interaction of the molten mass formed in the late stages of a [[nuclear accident]] (known as corium) with both water and [[concrete]]. | |||
===Chernobyl lava flow=== | |||
It is possible to see in the photo shown below that the corium (molten core) will cool and change to a solid with time. It is thought that the solid is weathering with time. The solid can be described as ''Fuel Containing Mass'', it is a mixture of sand, zirconium and uranium dixoide which had been heated at a very high temperature[http://ph.icmp.lviv.ua/chornobyl/e-library/tarapon-modeli_procesiv/Summary.htm] until it has melted. The chemical nature of this ''FCM'' has been the subject of some research.[http://www.maik.rssi.ru/abstract/radchem/1/radchem0596_abstract.pdf] The amount of fuel left in this form within the plant has been considered[http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=226794]. A [[Silicone]] polymer has been used to fix the contamination.[http://www.rense.com/general3/vhern.htm] | |||
The Chernobyl melt was a [[silicate]] melt which did contain inclusions of [[zirconium|Zr]]/[[uranium|U]] phases, moltern [[steel]] and high uranium [[zircon]]. The lava flow consists of more than one type of material a [[brown]] lava and a porous ceramic material have been found. | |||
The uranium to zirconium for different parts of the solid differs a lot, in the brown lava a uranium rich phase with a U:Zr ratio of 19:3 to about 38:10 is found. The uranium poor phase in the brown lava has a U:Zr ratio of about 1:10. S.V. Ushakov, B.E. Burakov, S.I. Shabalev and E.B. Anderson, ''Mater. Res. Soc. Symp. Proc.'', 1997, '''465''', 1313-1318.[http://www.thermo.ucdavis.edu/people/sergey/1.pdf] It is possible from the examination of the Zr/U phases to know the thermal history of the mixture, it can be shown that before the explosion that in part of the core the temperature was higher than 2000 <sup>o</sup>C. While in some areas the temperature was over 2400-2600 <sup>o</sup>C. | |||
===Water=== | |||
Extensive work was done from 1970 to 1990 on the possibility of a [[steam explosion]] or FCI when molten 'corium' contacted water. Many experiments suggested quite low conversion of thermal to mechanical energy, whereas the theoretical models available appeared to suggest that much higher efficiencies were possible. A [[NEA]]/[[OECD]] report was written on the subject in [[2000]] which states that a steam explosion caused by contact of corium corium with water has four stages.[http://www.nea.fr/html/nsd/docs/1999/csni-r99-24.pdf] | |||
* Premixing | |||
As the jet of corium enters the water, it breaks up into droplets. During this stage the thermal contact between the corium and the water is not good because a vapour film surrounds the droplets of corium and this insulates the two from each other. It is possible for this ''meta''-stable state to quench without an explosion or it can trigger in the next step | |||
* Triggering | |||
A externally or internally generated trigger (such as a pressure wave) causes a collapse of the vapour film between the corium and the water. | |||
* Propagation | |||
The local increase in pressure due to the increased heating of the water can generate enhanced heat transfer (usually due to rapid fragmentation of the hot fluid within the colder more volatile one) and a greater pressure wave, this process can be self-sustained. (The mechanics of this stage are similar to those in a classical ZND [[detonation wave]]). | |||
* Expansion | |||
This process leads to the whole of the water being suddenly heated to boiling. This causes an increase in pressure which can result in damage to the plant. | |||
==== Recent work ==== | |||
Some work has been done in Japan where [[uranium dioxide]] and [[zirconium]] dioxide was melted in a [[crucible]] before being added to [[water]]. The fragmentation of the fuel which results is reported in the paper [http://www.jstage.jst.go.jp/article/jnst/40/10/783/_pdf] which is in ''Journal of Nuclear Science and Technology[http://www.jstage.jst.go.jp/browse/jnst/_vols]'' | |||
===Concrete=== | |||
A review of the subject can be read at [http://www.nea.fr/html/nsd/docs/1987/csni87-143.pdf] and work on the subject continues to this day; in [[Germany]] at the [[FZK]] some work has been done on the effect of thermite on concrete, this is a simulation of the effect of the moltern core of a reactor breaking through the bottom of the pressure vessel into the containment.[http://www.ubka.uni-karlsruhe.de/vvv/fzk/6453/6453.text][http://bibliothek.fzk.de/zb/berichte/FZKA6453.pdf][http://bibliothek.fzk.de/zb/berichte/FZKA7002.pdf] | |||
[[Category:CZ Live]] | [[Category:CZ Live]] | ||
Revision as of 07:21, 25 December 2006
Post Irradiation Examination (PIE) and fuel behavior is a page devoted to the behaviour of nuclear fuel in a power reactor and the way in which used fuel is studied. It is common that experimental and production fuel will be examined after use in a reactor. [1][2] [3] [4] Due to the intensely radioactive nature of the used fuel this is done in a hot cell. A combination of nondestructive and destructive methods are used.
The PIE is used to check that the fuel is both safe and effective. After major accidents the core (or what is left of it) is normally sampled and then the samples are subjected to PIE in order to find out what happened. One site where PIE is done is the ITU which is the EU centre for the study of highly radioactive materials.
Swelling
It is normal for fuel to swell due to thermal expansion during use. A document on the subject can be downloaded from the NASA web site.[5]
Degradation of cladding
It is important to note that water and zirconium can react violently at 1200 oC, at the same temperature the zirconium cladding can react with uranium dioxide to form zirconium oxide and a uranium/zirconium alloy melt.[6] In normal use the metal can be damaged through neutron irradation, which tends to cause the metal to become brittle.
It is important to note that both the fuel can swell and the cladding which covers the fuel to form a fuel pin can be deformed. It is normal to fill the gap between the fuel and the cladding with helium gas to permit better thermal contact between the fuel and the cladding. During use the amount of gas inside the fuel pin can increase because of the formation of noble gases (krypton and xenon) by the fission process. If a Loss Of Coolant Accident (LOCA) {eg Three Mile Island or a Reactivity Initiated Accident (RIA) {eg Chernobyl or SL-1} occurs then the temperature of this gas can increase. As the fuel pin is sealed the presure of the gas will increase (PV = nRT) and it is possible to deform and burst the cladding. It has been noticed that both corrosion and irradiation can alter the properties of the zirconium alloy commonly used as cladding, making it brittle. As a result the experiments using unirradated zirconium alloy tubes can be misleading.
According to T. Nakamura, T. Fuketa, T. Sugiyama and H. Sasajima, Journal of Nuclear Science and Technology, 2004, 41, 37.[7] the following difference between the cladding failure mode of unused and used fuel was seen.
Unirradated fuel rods were pressurized before being placed in a special reactor at the Japanese Nuclear Safety Reasearch Reactor (NSRR) where they were subjected to a simulated RIA transient. These rods failed after ballooning late in the transient when the cladding temperature was high. The failure of the cladding in these tests was ductile, and it was a burst opening. The used fuel (61 GW days / ton of Uranium) failed early in the transient with a brittle fracture which was a longitundinal crack.
Experimental work on the degradation of fuel under accident conditions
A long series of experiments on the behaviour of fuel under accident conditions has been done at a range of different sites around the world, in some experiments a single part of the system is subjected to conditions which are an approximation of an accident while in other experiments a more complex system which simulates the behaviour of more than one part of the fuel/power reactor system is subjected to a simulated accident. In the following two series of experiments an entire fuel is subjected to conditions which approximate an accident. After the experiment the fuel (or wreckage) is subjected to PIE.
LOFT
The Loss of Fluid Tests (LOFT) were an early attempt to scope the response of real nuclear fuel to conditions under a Loss of Coolant Accident, funded by USNRC. The facility was built at Idaho National Laboratory, and was essentially a scale-model of a commercial PWR. ('Power/volume scaling' was used between the LOFT model, with a 50MWth core, and a commercial plant of 3000MWth).
The original intention (1963-1975) was to study only one or two major (large break) LOCA, since these had been the main concern of US 'rule-making' hearings in the late 1960s and early 1970s. These rules had focussed around a rather stylised large-break accident, and a set of criteria (eg for extent of fuel-clad oxidation) set out in 'Appendix K' of 10CFR50 (Code of Federal Regulations). However, following the accident at Three Mile Island, detailed modelling of much smaller LOCA became of equal concern.
38 LOFT tests were eventually performed and their scope was broadened to study a wide spectrum of breach sizes. These tests were used to help validate a series of computer codes (such as RELAP-4, RELAP-5 and TRAC) then being developed to calculate the thermal-hydraulics of LOCA.
- Some details of the tests can be read on-line.
PHEBUS
In France a facility exists in which a fuel melting incident can be made to happen under strictly controlled conditions.[18][19] In the PHEBUS research program fuels have been allowed to heat up to temperatures in excess of the normal operating temperatures, the fuel in question is in a special channel which is in a toroidal nuclear reactor. The nuclear reactor is used as a driver core to irradate the test fuel. While the reactor is cooled as normal by its own cooling system the test fuel has its own cooling system, which is fitted with filters and equipment to study the release of radioactivity from the damaged fuel. Already the release of radioisotopes from fuel under different conditions has been studied. After the fuel has been used in the experiment it is subject to a detailed examination (PIE), In the 2004 annual report from the ITU some results of the PIE on PHEBUS (FPT2) fuel are reported in section 3.6.[20][21]
Cracking of the fuel
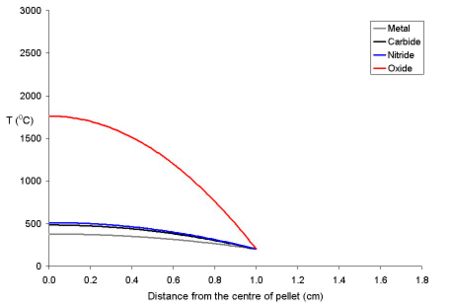
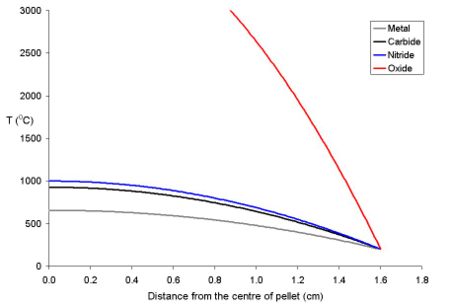
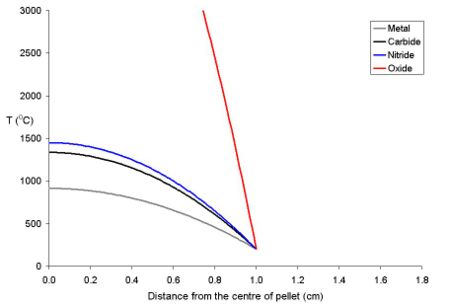
This is due to the fact that as the fuel expands on heating, the core of the pellet expands more than the rim. Because of the thermal stress thus formed the fuel cracks, the cracks tend to go from the centre to the edge in a star shaped pattern.
The temperature of the fuel varies as a function of the distance from the centre to the rim. At distance x from the centre the temperature (Tx) is described by the equation where ρ is the power density (W m-3) and Kf is the thermal conductivity.
- Tx = TRim + ρ (rpellet2 - x2) (4 Kf)-1
To explain this for a series of fuel pellets being used with a rim temperature of 200 oC (typical for a BWR) with different diameters and power densities of 250 Wm-3 have been modeled using the above equation. Note that these fuel pellets are rather large; it is normal to use oxide pellets which are about 10 mm in dimater.
Reference Radiochemistry and Nuclear Chemistry, G. Choppin, J-O Liljenzin and J. Rydberg, 3rd Ed, 2002, Butterworth-Heinemann, ISBN 0-7506-7463-6
Fission gas release
As the fuel is degraded or heated the more volatile fission products which are trapped within the uranium dioxide may become free. For example see J.Y. Colle, J.P. Hiernaut, D. Papaioannou, C. Ronchi, A. Sasahara, Journal of Nuclear Materials, 2006, 348, 229.
P. Wood and G.H. Bannister published a paper on the release of 85Kr, 106Ru and 137Cs from uranium when air is present. It was found that uranium dioxide was converted to U3O8 between about 300 and 500 oC in air. They report that this process requires some time to start, after the induction time the sample gains mass. The authors report that a layer of U3O7 was present on the uranium dioxide surface during this induction time. They report that 3 to 8% of the krypton-85 was released, and that much less of the ruthenium (0.5%) and cesium (2.6 x 10-3%) occurred during the oxidation of the uranium dioxide.[22]
The chemical nature of used uranium dioxide fuel
Overview
Used low enriched uranium nuclear fuel is an example of a nanomaterial which existed before the term nano became fashionable, in the oxide fuel intense temperature gradients exist which cause fission products to migrate. The zirconium tends to move to the centre of the fuel pellet where the temperature is highest while the lower boiling fission products move to the edge of the pellet. The pellet is likely to contain lots of small bubble like pores which form during use, the fission xenon migrates to these voids. Some of this xenon will then decay to form cesium, hence many of these bubbles contain a lot of Cs-137. Also metallic particles of an alloy of Mo-Tc-Ru-Pd tends to form in the fuel. Other solids form at the boundary between the uranium dioxide grains, but the majority of the fission products remain in the uranium dioxide as solid solutions.
For details of how to make a nonradioactive (uranium active) simulation of spent oxide fuel see: Microstructural features of SIMFUEL - Simulated high-burnup UO2-based nuclear fuel, P.G. Lucuta, R.A. Verrall, Hj. Matzke and B.J. Palmer, Journal of Nuclear Materials, 1991, 178, 48-60. This SIMFUEL has been used in a large number of experiments in which attempts are made to predict the long term behaviour of used nuclear fuels within a waste store. One leading worker in this area is David Shoesmith[23][24] who is an electrochemist working in Canada, he tends to use many of the electrochemical experiments normally used for the study of galvanic corrosion to investigate the galvanic of uranium dioxide.
According to Dave Shoesmith's work the nanoparticles of Mo-Tc-Ru-Pd have a strong effect on the corrosion of uranium dioxide fuel. He suggests that when the hydrogen (H2) concentration is high (due to the anaerobic corrosion of the steel waste can) the oxidation of hydrogen at the nanoparticles will exert a protective effect on the uranium dioxide. This effect can be thought of as an example of protection by a sacrificial anode where instead of a metal anode reacting and dissolving it is the hydrogen gas which is consumed.
Uranium
- 96% of the mass is the remaining uranium: most of the original 238U and a little 235U. Usually 235U would be less than 0.83% of the mass along with 0.4% 236U.
Plutonium
- 1% of the mass is 239Pu and 240Pu resulting from conversion of 238U, which may either be considered a useful by-product, or as dangerous and inconvenient waste. One of the main concerns regarding nuclear proliferation is to prevent this plutonium from being used by states other than those already established as Nuclear Weapons States, to produce nuclear weapons. If the reactor has been used normally, the plutonium is reactor-grade, not weapon-grade: it contains much 240Pu and less than 80% 239Pu, which makes it less suitable, but not impossible, to use in a weapon [25]. If the irradiation period has been short then the plutonium is weapon-grade (more than 80%, up to 93%).
Minor actinides
- Traces of the minor actinides are present in used reactor fuel. These are actinides other than uranium and plutonium. These include americium and curium. The amount formed depends greatly upon the nature of the fuel used and the conditions under which it was used. For instance the use of MOX fuel (239Pu in a 238U matrix) is likely to lead to the production of more 241Am than the use of a uranium/thorium based fuel (233U in a 232Th matrix). Also present as a minor actinide is 237Np, this neptunium isotope is fissile but also can be converted into 238Pu by neutron bombardment.
Fission products
- 3% of the mass consists of fission products of 235U (also indirect products in the decay chain), nuclear poisons considered radioactive waste or separated further for various industrial and medical uses. The fission products include every element from zinc through to the lanthanides, much of the fission yield is concentrated in two peaks, one in the second transition row (Zr, Mo, Tc, Ru, Rh, Pd, Ag) while the other is later in the periodic table (I, Xe, Cs, Ba, La, Ce, Nd). Many of the fission products are either non radioactive or only shortly lived radioisotopes. But a considerable number are medium to long lived radioisotopes such as 90Sr, 137Cs, 99Tc and 129I. Research has been conducted by several different countries into partitioning the rare isotopes in fission waste including the Fission Platinoids (Ru, Rh, Pd) and Silver (Ag) as a way of offsetting the cost of reprocessing, however this is not currently being done commercially.
A report has been written which contains estimates of the amounts of different isotopes emitted during the chernobyl fire, the fire and explosion at chernobyl is one of the largest releases of radioactivity into the environment which has occured and hence deserves special attention(OECD NEA report on Chernobyl {ten years on}[26])
| Element | Gas | Metal | Oxide | Solid solution | Radioisotopes | Release at Chernobyl[28] | T required for 10% release from UO2 | T required for 10% release from U3O8 |
|---|---|---|---|---|---|---|---|---|
| Br | Yes | - | - | - | - | - | - | - |
| Kr | Yes | - | - | - | 85Kr | 100% | - | - |
| Rb | Yes | - | Yes | - | - | - | - | - |
| Sr | - | - | Yes | Yes | 89Sr and 90Sr | 4-6% | 1950 K | - |
| Y | - | - | - | Yes | - | 3.5% | - | - |
| Zr | - | - | Yes | Yes | 95Zr | 3.5% | 2600 K | - |
| Nb | - | - | Yes | - | - | - | - | - |
| Mo | - | Yes | Yes | - | 99Mo | >3.5% | - | 1200 K |
| Tc | - | Yes | - | - | - | - | - | 1300 K |
| Ru | - | Yes | - | - | 103Ru and 106Ru | >3.5% | - | - |
| Rh | - | Yes | - | - | - | - | - | - |
| Pd | - | Yes | - | - | - | - | - | - |
| Ag | - | Yes | - | - | - | - | - | - |
| Cd | - | Yes | - | - | - | - | - | - |
| In | - | Yes | - | - | - | - | - | - |
| Sn | - | Yes | - | - | - | - | - | - |
| Sb | - | Yes | - | - | - | - | - | - |
| Te | Yes | Yes | Yes | Yes | 132Te | 25-60% | 1400 K | 1200 K |
| I | Yes | - | - | - | 131I | 50-60% | 1300 K | 1100 K |
| Xe | Yes | - | - | - | 133Xe | 100% | 1450 K | - |
| Cs | Yes | - | Yes | - | 134Cs and 137Cs | 20-40% | 1300 K | 1200 to 1300 K |
| Ba | - | - | Yes | Yes | 140Ba | 4-6% | 1850 K | 1300 K |
| La | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Ce | - | - | - | Yes | 141Ce and 144Ce | 3.5% | 2300 K | - |
| Pr | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Nd | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Pm | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Sm | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Eu | - | - | - | Yes | - | 3.5% | 2300 K | - |
J.Y. Colle, J.-P. Hiernaut, D. Papaioannou, C. Ronchi and A. Sasahara, Journal of Nuclear Materials, 2006, 348, 229-242 reported the releases of fission products and uranium from uranium dioxide (from spent BWR fuel, burn-up was 65 GWd t-1) which was heated in a Knudsen cell. Fuel was heated in the Knudsen cell both with and without preoxidation in oxygen at c 650 K. It was found even for the noble gases that a high temperature was required to liberate them from the uranium oxide solid. For unoxidized fuel 2300 K was required to release 10% of the uranium while oxidized fuel only requires 1700 K to release 10% of the uranium.
According to the report on Chernobyl used in the above table 3.5% of the following isotopes in the core were released 239Np, 238Pu, 239Pu , 240Pu, 241Pu and 242Cm.
Contact of molten fuel with water and concrete
While much of this work is strictly PIE it is important to understand the interaction of the molten mass formed in the late stages of a nuclear accident (known as corium) with both water and concrete.
Chernobyl lava flow
It is possible to see in the photo shown below that the corium (molten core) will cool and change to a solid with time. It is thought that the solid is weathering with time. The solid can be described as Fuel Containing Mass, it is a mixture of sand, zirconium and uranium dixoide which had been heated at a very high temperature[29] until it has melted. The chemical nature of this FCM has been the subject of some research.[30] The amount of fuel left in this form within the plant has been considered[31]. A Silicone polymer has been used to fix the contamination.[32]
The Chernobyl melt was a silicate melt which did contain inclusions of Zr/U phases, moltern steel and high uranium zircon. The lava flow consists of more than one type of material a brown lava and a porous ceramic material have been found. The uranium to zirconium for different parts of the solid differs a lot, in the brown lava a uranium rich phase with a U:Zr ratio of 19:3 to about 38:10 is found. The uranium poor phase in the brown lava has a U:Zr ratio of about 1:10. S.V. Ushakov, B.E. Burakov, S.I. Shabalev and E.B. Anderson, Mater. Res. Soc. Symp. Proc., 1997, 465, 1313-1318.[33] It is possible from the examination of the Zr/U phases to know the thermal history of the mixture, it can be shown that before the explosion that in part of the core the temperature was higher than 2000 oC. While in some areas the temperature was over 2400-2600 oC.
Water
Extensive work was done from 1970 to 1990 on the possibility of a steam explosion or FCI when molten 'corium' contacted water. Many experiments suggested quite low conversion of thermal to mechanical energy, whereas the theoretical models available appeared to suggest that much higher efficiencies were possible. A NEA/OECD report was written on the subject in 2000 which states that a steam explosion caused by contact of corium corium with water has four stages.[34]
- Premixing
As the jet of corium enters the water, it breaks up into droplets. During this stage the thermal contact between the corium and the water is not good because a vapour film surrounds the droplets of corium and this insulates the two from each other. It is possible for this meta-stable state to quench without an explosion or it can trigger in the next step
- Triggering
A externally or internally generated trigger (such as a pressure wave) causes a collapse of the vapour film between the corium and the water.
- Propagation
The local increase in pressure due to the increased heating of the water can generate enhanced heat transfer (usually due to rapid fragmentation of the hot fluid within the colder more volatile one) and a greater pressure wave, this process can be self-sustained. (The mechanics of this stage are similar to those in a classical ZND detonation wave).
- Expansion
This process leads to the whole of the water being suddenly heated to boiling. This causes an increase in pressure which can result in damage to the plant.
Recent work
Some work has been done in Japan where uranium dioxide and zirconium dioxide was melted in a crucible before being added to water. The fragmentation of the fuel which results is reported in the paper [35] which is in Journal of Nuclear Science and Technology[36]
Concrete
A review of the subject can be read at [37] and work on the subject continues to this day; in Germany at the FZK some work has been done on the effect of thermite on concrete, this is a simulation of the effect of the moltern core of a reactor breaking through the bottom of the pressure vessel into the containment.[38][39][40]