User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok |
||
| Line 10: | Line 10: | ||
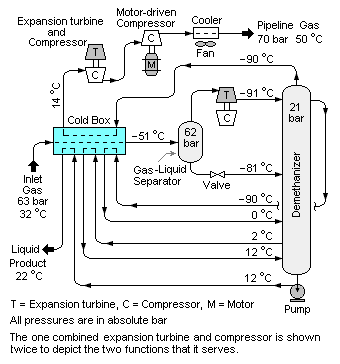
[[Image:Demethanizer.PNG|right|thumb|350px|{{#ifexist:Template:Demethanizer.PNG/credit|{{Demethanizer.PNG/credit}}<br/>|}}Fig. 2: A schematic diagram of a demethanizer extracting hydrocarbon liquids from natural gas.]] | [[Image:Demethanizer.PNG|right|thumb|350px|{{#ifexist:Template:Demethanizer.PNG/credit|{{Demethanizer.PNG/credit}}<br/>|}}Fig. 2: A schematic diagram of a demethanizer extracting hydrocarbon liquids from natural gas.]] | ||
Although expansion turbines are | Although expansion turbines are very commonly used in low-temperature processes, they are used in many other applications as well. This section discusses one of the low temperature procesees as well as some of the other applications. | ||
===Extracting hydrocarbon liquids from natural gas=== | |||
Raw natural gas consists primarily of methane (CH<sub>4</sub>), the shortest and lightest [[hydrocarbon]] molecule. It also contains varying amounts of heavier gaseous hydrocarbons such as [[ethane]] (C<sub>2</sub>H<sub>6</sub>), [[propane]] (C<sub>3</sub>H<sub>8</sub>), [[Butane|normal butane]] (n-C<sub>4</sub>H<sub>10</sub>), [[isobutane]] (i-C<sub>4</sub>H<sub>10</sub>), [[pentane]]s and even higher [[molecular weight]] hydrocarbons. When processed and purified into finished by-products, all of these are collectively referred to '''NGL (Natural Gas Liquids)'''. It also contains *[[Acid gas]]es: [[carbon dioxide]] (CO<sub>2</sub>), [[hydrogen sulfide]] (H<sub>2</sub>S) and [[mercaptan]]s such as [[methanethiol]] (CH<sub>3</sub>SH) and [[ethanethiol]] {C<sub>2</sub>H<sub>5</sub>SH). | |||
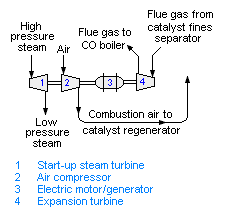
[[Image:Fluid catalytic cracker power recovery.png|right|thumb|350px|{{#ifexist:Template:Fluid catalytic cracker power recovery.png/credit|{{Fluid catalytic cracker power recovery.png/credit}}<br/>|}}Fig. 3: A schematic diagram of the power recovery system in a fluid catalytic cracking unit.]] | [[Image:Fluid catalytic cracker power recovery.png|right|thumb|350px|{{#ifexist:Template:Fluid catalytic cracker power recovery.png/credit|{{Fluid catalytic cracker power recovery.png/credit}}<br/>|}}Fig. 3: A schematic diagram of the power recovery system in a fluid catalytic cracking unit.]] | ||
Revision as of 13:40, 10 July 2008
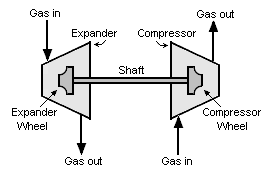
An expansion turbine, also referred to as a turboexpander or turbo-expander, is a centrifugal or axial flow turbine through which a high pressure gas is expanded to produce work that is often used to drive a gas compressor.
Because work is extracted from the expanding high pressure gas, the expansion is an isentropic process (i.e., a constant entropy process) and the low pressure exhaust gas from the turbine is at a very low temperature, sometimes as low as -90 °C or less.
Expansion turbines are very widely used as sources of refrigeration in industrial processes such as the extraction of ethane and natural gas liquids (NGLs) from natural gas,[1] the liquefaction of gases (such as oxygen, nitrogen, helium, argon and krypton)[2][3] and other low-temperature processes.
Applications
Although expansion turbines are very commonly used in low-temperature processes, they are used in many other applications as well. This section discusses one of the low temperature procesees as well as some of the other applications.
Extracting hydrocarbon liquids from natural gas
Raw natural gas consists primarily of methane (CH4), the shortest and lightest hydrocarbon molecule. It also contains varying amounts of heavier gaseous hydrocarbons such as ethane (C2H6), propane (C3H8), normal butane (n-C4H10), isobutane (i-C4H10), pentanes and even higher molecular weight hydrocarbons. When processed and purified into finished by-products, all of these are collectively referred to NGL (Natural Gas Liquids). It also contains *Acid gases: carbon dioxide (CO2), hydrogen sulfide (H2S) and mercaptans such as methanethiol (CH3SH) and ethanethiol {C2H5SH).
Power recovery in fluid catalytic cracker
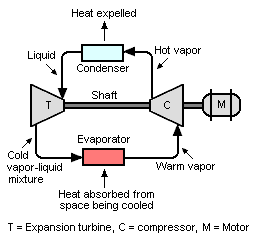
Refrigeration system
Power generation
History
In 1939, Pyotr Kapitza of Russia suggested the use of a centrifugal turbine for the isentropic expansion of gases to produce refrigeration. Since then, centrifugal expansion turbines have taken over almost 100 percent of the gas liquefaction and other low-temperature industrial requirements.
References
- ↑ Demethanzer
- ↑ BOC (NZ) publication: use search function for keyword "expansion"
- ↑ US Department of Energy Hydrogen Program