User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok No edit summary |
||
| Line 4: | Line 4: | ||
Condensate polishing is a unique application of [[ion-exchange resin]]s that removes suspended and dissolved impurities from the condensate. It is essential for the very stringent quality required of high-pressure steam generation feedwater. | Condensate polishing is a unique application of [[ion-exchange resin]]s that removes suspended and dissolved impurities from the condensate. It is essential for the very stringent quality required of high-pressure steam generation feedwater. | ||
== | ==The ion exchange process mechanism== | ||
{{Image|Ion Exchange.PNG|right|322px|Diagram depicting how ion exchange resins work.}} | {{Image|Ion Exchange.PNG|right|322px|Diagram depicting how ion exchange resins work.}} | ||
Ion exchange is a reversible chemical reaction wherein [[ion]]s ([[atom]]s or [[molecule]]s that have lost or gained an [[electron]] and thus acquired an electrical charge) present in a solution are exchanged for a similarly charged ion attached to an immobile solid particle of ion exchange material. | Ion exchange is a reversible chemical reaction wherein [[ion]]s ([[atom]]s or [[molecule]]s that have lost or gained an [[electron]] and thus acquired an electrical charge) present in a solution are exchanged for a similarly charged ion attached to an immobile solid particle of ion exchange material. | ||
By charging (i.e., treating) ion exchange material with an [[acid]], usually [[sulfuric acid]] (H<sub>2</sub>SO<sub>4</sub>) or [[hydrochloric acid]] (HCl), positively charged hydrogen ions (H<sup>+</sup>) are attached to sites on the ion exchange material. Those | By charging (i.e., treating) ion exchange material with an [[acid]], usually [[sulfuric acid]] (H<sub>2</sub>SO<sub>4</sub>) or [[hydrochloric acid]] (HCl), positively charged hydrogen ions (H<sup>+</sup>) are attached to sites on the ion exchange material. Those H<sup>+</sup> ions will attract and exchange places with other positively charged ions (called ''cations'') present in a solution brought into contact with the positively charged H<sup>+</sup> ions. | ||
Similarly, by charging ion exchange material with a [[caustic]] such as [[sodium hydroxide]] (NaOH), negatively charged hydroxide ions (OH<sup>-</sup>) are attached to sites on the ion exchange material. Those sites will attract other negatively charged ions (called ''anions'') present in a solution brought into contact with the negatively charged OH<sup>-</sup> ions. | Similarly, by charging ion exchange material with a [[caustic]] such as [[sodium hydroxide]] (NaOH), negatively charged hydroxide ions (OH<sup>-</sup>) are attached to sites on the ion exchange material. Those sites will attract and exchange places with other negatively charged ions (called ''anions'') present in a solution brought into contact with the negatively charged OH<sup>-</sup> ions. | ||
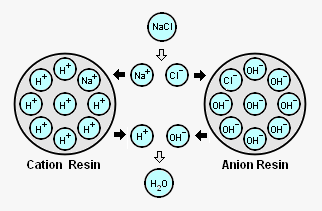
The adjacent diagram schematically depicts an acid treated bead of ion exchange resin with its attached H<sup>+</sup> ions (referred to as ''cation resin'') and a caustic treated bead of ion exchange resin with its attached OH<sup>-</sup> ions (referred to as an "anion resin"). | The adjacent diagram schematically depicts an acid treated bead of ion exchange resin with its attached H<sup>+</sup> ions (referred to as ''cation resin'') and a caustic treated bead of ion exchange resin with its attached OH<sup>-</sup> ions (referred to as an "anion resin"). As shown in the diagram, when those beads are contacted with [[sodium]] cations (Na<sup>+</sup>) and [[chlorine]] anions (Cl<sup>-</sup>) derived from dissolved [[sodium chloride]] in solution: | ||
* The Na<sup>+</sup> cations are attracted to the cation resin and exchange places with the H<sup>+</sup> cations on the resin. | |||
* The Cl<sup>-</sup> anions are attracted to the anion resin and exchange place with the OH<sup>-</sup> anions on the resin. | |||
* The H<sup>+</sup> cations removed from the cation resin and the OH<sup>-</sup> anions removed from the anion resin combine with each other to form a molecule of water that has no charge. | |||
That illustrates how ion exchange material removes dissolved substances from steam condensate or any other solution. | |||
Once all of the charged ions attached on the cation and anion exchange materials have been replaced by ions removed from a solution, the ion exchange materials are refered to as ''spent''. The spent materals can be regenerated (i.e., rejuvenated) by once again being charged with acid or caustic. | |||
==Types of condensate polishing== | |||
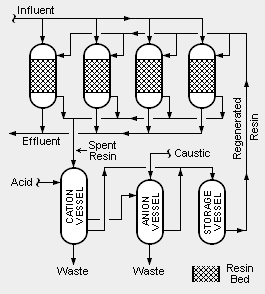
{{Image|Condensate Polisher.png|right|265px|Schematic diagram of a mixed-bed condensate polisher.}} | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 13:16, 6 August 2009
Condensate polishing is a process used to purify the steam condensate produced in high-pressure steam generation facilities[1][2][3][4] such as those in large thermal power plants.[5] Steam condensate is the water formed by condensing the exhaust steam from the steam-driven turbines in thermal power plants and which is recycled for reuse as the major part of the steam generation feedwater.
Condensate polishing is a unique application of ion-exchange resins that removes suspended and dissolved impurities from the condensate. It is essential for the very stringent quality required of high-pressure steam generation feedwater.
The ion exchange process mechanism
Ion exchange is a reversible chemical reaction wherein ions (atoms or molecules that have lost or gained an electron and thus acquired an electrical charge) present in a solution are exchanged for a similarly charged ion attached to an immobile solid particle of ion exchange material.
By charging (i.e., treating) ion exchange material with an acid, usually sulfuric acid (H2SO4) or hydrochloric acid (HCl), positively charged hydrogen ions (H+) are attached to sites on the ion exchange material. Those H+ ions will attract and exchange places with other positively charged ions (called cations) present in a solution brought into contact with the positively charged H+ ions.
Similarly, by charging ion exchange material with a caustic such as sodium hydroxide (NaOH), negatively charged hydroxide ions (OH-) are attached to sites on the ion exchange material. Those sites will attract and exchange places with other negatively charged ions (called anions) present in a solution brought into contact with the negatively charged OH- ions.
The adjacent diagram schematically depicts an acid treated bead of ion exchange resin with its attached H+ ions (referred to as cation resin) and a caustic treated bead of ion exchange resin with its attached OH- ions (referred to as an "anion resin"). As shown in the diagram, when those beads are contacted with sodium cations (Na+) and chlorine anions (Cl-) derived from dissolved sodium chloride in solution:
- The Na+ cations are attracted to the cation resin and exchange places with the H+ cations on the resin.
- The Cl- anions are attracted to the anion resin and exchange place with the OH- anions on the resin.
- The H+ cations removed from the cation resin and the OH- anions removed from the anion resin combine with each other to form a molecule of water that has no charge.
That illustrates how ion exchange material removes dissolved substances from steam condensate or any other solution.
Once all of the charged ions attached on the cation and anion exchange materials have been replaced by ions removed from a solution, the ion exchange materials are refered to as spent. The spent materals can be regenerated (i.e., rejuvenated) by once again being charged with acid or caustic.
Types of condensate polishing
References
- ↑ Larry Drbal, Kayla Westra and Pat Boston (1996). Power Plant Engineering, 1st Edition. Springer. ISBN 0-412-06401-4.
- ↑ Brad Buecker (2000). Fundamentals of Steam Generation Chemistry, 1st Edition. Penwell. ISBN 0-87814-750-0.
- ↑ Condensate Polishing Guidelines Electric Power Research Institute (EPRI), 1996
- ↑ Condensate Polishing State of Knowledge Assessment Electric Power Research Institute (EPRI), 2006
- ↑ Either nuclear or fuel-fired power plants