User:Milton Beychok/Sandbox: Difference between revisions
Jump to navigation
Jump to search
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 2: | Line 2: | ||
{| class = "wikitable" align="left" | {| class = "wikitable" align="left" | ||
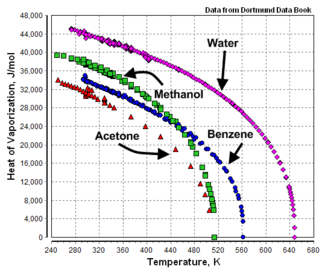
|+ Heat of vaporization, boiling point, and critical | |+ Heat of vaporization, normal boiling point, and critical<br/>points of various liquids | ||
! Name!!Formula!!H<sub>v</sub><br/>(J/mol) | !Name!!Formula!!H<sub>v</sub><br/>( J/mol )!!T<sub>n</sub><br/>( °C )!!T<sub>n</sub><br/>( K )!!T<sub>c</sub><br/>( K )!!P<sub>c</sub><br/>( atm ) | ||
|- align="center" | |||
|Acetic acid||C<sub>2</sub>H<sub>4</sub>O<sub>2</sub>||23,700||117.9||391.1||594.8||57.1 | |||
|- align="center" | |- align="center" | ||
| Acetone || C<sub>3</sub>H<sub>6</sub>O||29,100||329.4|| | | Acetone||C<sub>3</sub>H<sub>6</sub>O||29,100||56.2||329.4||508.7||47.0 | ||
|- align="center" | |||
|Benzene||C<sub>6</sub>H<sub>6</sub>||30,720||80.0||353.2||562.1||48.6 | |||
|} | |} | ||
Revision as of 01:48, 7 September 2009
| Name | Formula | Hv ( J/mol ) |
Tn ( °C ) |
Tn ( K ) |
Tc ( K ) |
Pc ( atm ) |
|---|---|---|---|---|---|---|
| Acetic acid | C2H4O2 | 23,700 | 117.9 | 391.1 | 594.8 | 57.1 |
| Acetone | C3H6O | 29,100 | 56.2 | 329.4 | 508.7 | 47.0 |
| Benzene | C6H6 | 30,720 | 80.0 | 353.2 | 562.1 | 48.6 |