3-hydroxy-3-methyl-glutaryl CoA: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (New page: {{subpages}} [[Image:Mevalonate synthesis.jpg|right|thumb|250px|{{#ifexist:Template:Mevalonate synthesis.jpg/credit|{{Mevalonate synthesis.jpg/credit}}<br/>|}}Biosynthesis of mevalonate fr...) |
imported>David E. Volk mNo edit summary |
||
| Line 2: | Line 2: | ||
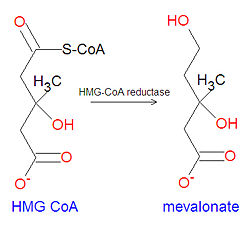
[[Image:Mevalonate synthesis.jpg|right|thumb|250px|{{#ifexist:Template:Mevalonate synthesis.jpg/credit|{{Mevalonate synthesis.jpg/credit}}<br/>|}}Biosynthesis of mevalonate from HMG CoA.]] | [[Image:Mevalonate synthesis.jpg|right|thumb|250px|{{#ifexist:Template:Mevalonate synthesis.jpg/credit|{{Mevalonate synthesis.jpg/credit}}<br/>|}}Biosynthesis of mevalonate from HMG CoA.]] | ||
The biochemical '''3-hydroxy-3-methyl-glutaryl CoA''' | The biochemical '''3-hydroxy-3-methyl-glutaryl CoA''' '''(HMG CoA)''', is a chemical precursor of [[mevalonate]], which is a key metabolite in the synthesis of [[cholesterol]]. The cholesterol-lowering statins, such as [[Lipitor]] and [[Crestor]], among others, are [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitor]] which block the synthesis of mevalonate from HMG CoA. | ||
Revision as of 13:29, 24 January 2008
The biochemical 3-hydroxy-3-methyl-glutaryl CoA (HMG CoA), is a chemical precursor of mevalonate, which is a key metabolite in the synthesis of cholesterol. The cholesterol-lowering statins, such as Lipitor and Crestor, among others, are HMG-CoA reductase inhibitor which block the synthesis of mevalonate from HMG CoA.