Stent: Difference between revisions
imported>Howard C. Berkowitz m (fixed PTCA link) |
imported>Robert Badgett |
||
| Line 12: | Line 12: | ||
== Drug eluting stents == | == Drug eluting stents == | ||
Drug-eluting stents further reduce restenosis compared with bare-metal stents<ref name="pmid16971716">{{cite journal |author=Spaulding C, Henry P, Teiger E, ''et al'' |title=Sirolimus-eluting versus uncoated stents in acute myocardial infarction |journal=N. Engl. J. Med. |volume=355 |issue=11 |pages=1093–104 |year=2006 |month=September |pmid=16971716 |doi=10.1056/NEJMoa062006 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=16971716&promo=ONFLNS19 |issn=}}</ref>, but drug-eluting stents may increase the rate of delayed restenoses. Delayed restenosis may be prevented by taking [[aspirin]] combined with [[clopidogrel]].<ref name="pmid17202455"/> | |||
The drugs eluted are [[sirolimus]] and [[paclitaxel]]. Sirolimus was first approved in the [[United States]] by the [[Food and Drug Administration]] in 2003.<ref name="urlDevices@FDA11506 ">{{cite web |url=http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=PMA&id=11506 |title=Devices@FDA |author=Anonymous |authorlink= |coauthors= |date=2003 |format= |work= |publisher=Food and Drug Administration |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-05-02}}</ref> Paclitaxel was first approved in the [[United States]] in 2004.<ref name="urlDevices@FDA14019">{{cite web |url=http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=PMA&id=14019 |title=Devices@FDA |author=Anonymous |authorlink= |coauthors= |date=2004 |format= |work= |publisher=Food and Drug Administration |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-05-02}}</ref> These stents have become one of the best selling devices in medical history, and the worldwide market for drug-eluting coronary stents reached an estimated $4.2 billion in 2004 and is expected to nearly double by 2010.<ref>{{cite web |url=http://www.devicelink.com/ | title=Medical Device Link, Jan./Feb. 2006}}</ref> | |||
Two drug eluting stents have dominated the market: the [[Taxus]]<ref>{{cite web |url=http://www.taxus-stent.com/ | title = Taxus stent webpage}}</ref> stent from [[Boston Scientific]] and the [[Cypher]]<ref>{{cite web |url=http://www.cypherusa.com/cypher-j2ee/cypherjsp/main_splash/index.jsp | title=Cypher stent webpage}}</ref> stent from [[Cordis]], a [[Johnson and Johnson]] company. Taxus elutes the drug [[paclitaxel]], while Cypher elutes [[sirolimus]]. | Two drug eluting stents have dominated the market: the [[Taxus]]<ref>{{cite web |url=http://www.taxus-stent.com/ | title = Taxus stent webpage}}</ref> stent from [[Boston Scientific]] and the [[Cypher]]<ref>{{cite web |url=http://www.cypherusa.com/cypher-j2ee/cypherjsp/main_splash/index.jsp | title=Cypher stent webpage}}</ref> stent from [[Cordis]], a [[Johnson and Johnson]] company. Taxus elutes the drug [[paclitaxel]], while Cypher elutes [[sirolimus]]. | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 11:09, 27 May 2009
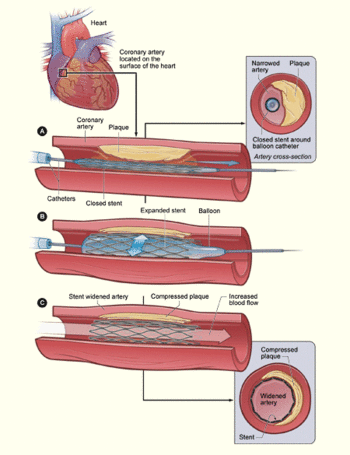
A coronary stent is a small, lattice-shaped, metal tube that is inserted permanently into an artery. The stent helps hold open an artery so that blood can flow through it.[1] It is often used for patients suffering heart attacks or atherosclerosis, a condition in which the arteries become blocked due to plaque build up on the inner walls of arteries, the blood vessels that carry oxygen-rich blood throughout the body. A stent restores blood flow by acting as a permenant scaffold to hold the artery open.
Insertion
Stents are typically inserted in the femoral artery in the groin or the brachial artery in the arm and threaded up to the narrowed artery section using a balloon catheter. When the blockage is reached, the balloon is inflated to push the plaque out of the way, expanding the artery. This is referred to as percutaneous transluminal coronary angioplasty (PTCA). Expandable stents are opened as the balloon expands, while other stents are inserted immediately after the PTCA is completed. During the procedure, contrast dye is typically running through the arterial system to guide the insertion of the stent. Following the procedure, the patient must take anti-platelet drugs to avoid thrombosis. Some stents are coated with a time released drug to prevent blod clots. Because stents are inserted into major arteries, a great deal of pressure must be applied to the insertion site to avoid excess bleeding, particularly since the patient has typically been given anti-platelet medications during the procedure.
Risks
Infection, blood clots, or bleeding may occur after stent insertion. Other rare complications of coronary stents include chest pain, heart attack, or tearing of the blood vessel. The stent can move out of place (stent migration). In some cases, plaque can reappear in the stented artery (in-stent restenosis). Drug eluting stents (DES) have additional risks which are being monitored by the FDA since their introduction in 2003-2004.
Drug eluting stents
Drug-eluting stents further reduce restenosis compared with bare-metal stents[2], but drug-eluting stents may increase the rate of delayed restenoses. Delayed restenosis may be prevented by taking aspirin combined with clopidogrel.[3]
The drugs eluted are sirolimus and paclitaxel. Sirolimus was first approved in the United States by the Food and Drug Administration in 2003.[4] Paclitaxel was first approved in the United States in 2004.[5] These stents have become one of the best selling devices in medical history, and the worldwide market for drug-eluting coronary stents reached an estimated $4.2 billion in 2004 and is expected to nearly double by 2010.[6]
Two drug eluting stents have dominated the market: the Taxus[7] stent from Boston Scientific and the Cypher[8] stent from Cordis, a Johnson and Johnson company. Taxus elutes the drug paclitaxel, while Cypher elutes sirolimus.
References
- ↑ FDA stent webpage.
- ↑ Spaulding C, Henry P, Teiger E, et al (September 2006). "Sirolimus-eluting versus uncoated stents in acute myocardial infarction". N. Engl. J. Med. 355 (11): 1093–104. DOI:10.1056/NEJMoa062006. PMID 16971716. Research Blogging.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid17202455 - ↑ Anonymous (2003). Devices@FDA. Food and Drug Administration. Retrieved on 2009-05-02.
- ↑ Anonymous (2004). Devices@FDA. Food and Drug Administration. Retrieved on 2009-05-02.
- ↑ Medical Device Link, Jan./Feb. 2006.
- ↑ Taxus stent webpage.
- ↑ Cypher stent webpage.