Aspergillus niger: Difference between revisions
imported>Alice Chang No edit summary |
imported>Alice Chang No edit summary |
||
| Line 20: | Line 20: | ||
| binomial = ''Aspergillus niger'' | | binomial = ''Aspergillus niger'' | ||
| binomial_authority = | | binomial_authority = | ||
[ | [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=425011&lvl=3&lin=f&keep=1&srchmode=1&unlock] | ||
}} | }} | ||
Revision as of 05:21, 12 May 2009
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is May 21, 2009. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
| Aspergillus niger | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||||||||||
| Scientific classification | ||||||||||||||||||||||
| ||||||||||||||||||||||
| Binomial name | ||||||||||||||||||||||
| Aspergillus niger [1] |
Description and significance
Aspergillus niger is a filamentous fungus that has many applications in biotechnology.[1]
This eukaryotic organism belongs to the Fungi kingdom and the Aspergillus genus. The use of microorganisms in biotechnology is common, however A. niger is considered to be one of the most essential of those microorganisms.[2]
The significance of A. niger is the industrial role that it plays in the production of proteins, enzymes and fermentation. A. niger strain ATCC 1015 is most well-known as the strain that produces citric acid. Citric acid serves the purpose of improving taste, nutrition and shelf-life of food products in the food industry.
Industrial workhorse.[3]
Proteins[4]
Genome structure
The genome sequencing of Aspergillus niger can be performed with many advanced biological analysis techniques. Continued research in this microbe could lead to greater possibilities for the use of A. niger; as there are 7,500 genes that are not yet known.[5]
A. niger CBS 513.88 strain is the early ancestor of enzyme production strains that are currently used. CBS 513.88 strain was sequenced by large team that used a process called BAC walking, which required the use of an ordered set of large E. coli bacterial artificial chromosomes (BACs). The results from the study were published in January 2007 in Nature Biotechnology. Results indicated that the size of the genome of A. niger CBS 513.88 is 33.9-megabased with a total of 14,165 protein-coding genes. The assembled genome sequence expresses 468 DNA contigs, a set of overlapping DNA segments that stretch a total of 33.9 million unique bp (Mbp) that are arranged in 19 supercontigs. The average gene length is 1,572 bp and the number of tRNA genes is 269.[5]
"Table 1. Genome characteristics of A. niger CBS 513.88"[5]
| Size assembled genome (Mb) | 33.9 |
| GC content assembled genome (%) | 50.4 |
| Protein-coding genes (all) | 14,165 |
| Protein-coding genes (<100 aa) | 927 |
| GC content protein-coding genes (%) | 53.4 |
| Average gene length (bp) | 1,572 |
| Gene density (genes/kb) | 0.42 |
| Average number of introns per gene | 2.57 |
| Average intron size (bp) | 97 |
| Average exon size (bp) | 370 |
| Number of tRNA genes | 269 |
Cell structure and metabolism
A. niger is a fungus, but it is specified as a mold.
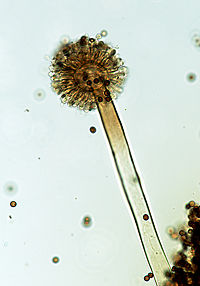
Therefore, A. niger takes on many of the common features that a microscopic fungi which grows in a filamentous form that are multicellular and called hyphae. Those microscopic fungi that are not multicellular, but are single celled fungi are known as yeasts. These hyphae that appear as an connected network that are tube-shaped, branched contain nuclei that are identical genetically nuclei, can be considered as a single organism, which are called a colony or a mycelium. Under a microscope, the appearance of the hyphae of A. niger are septate, meaning that it is separated by a septum; they are colorless and have conidial heads which are radial at first but with time, division into column structure occurs. Conidia are spherical in shape and brownish-black in color with a rough texture.
Ecology
A. niger grows aerobically on organic matter, therefore it can be found almost everywhere in environments that contain soil. Also, it is found in waste, decaying plant material and compost in outdoor environments. Moist indoor environments create a good habitat for the growth of mold. There are other indoor molds that also appear as black spores, which have similar resemblance to A. niger.[7]
Aspergillus niger has been exhibited to sustain growth in freezing temperatures, which indicates it as a thermotolerant that can also survive at very high temperatures. It’s thermotolerant abilities that enable growth in a wide temperature range from 6 to 47°C with a preferred optimum temperature at 35-37°C. The fungus is capable of growing over a very wide pH range, from 1.4 to 9.8 pH. The growth ability in various temperature ranges, pH ranges as well as the abundant amount of conidiospores allow the species to be continuously widespread. Conidiospores are distributed by air.[2]
Plant pathology
In plants, the Black Rot disease affects onions when black-colored spores of A. niger are present, appearance of black mold of the onions. This filamentous fungus causes diseases in peanuts and in grapes as well.[8]
Human and Animal interactions
Application to Biotechnology
In the field of biotechnology, A. niger is a very useful and important microorganism because it has the ability to produce any substances. It is therefore cultured for industrial production. Many enzymes, ie. citric acid, amylases, lipases, cellulose, xylanases and proteases are produced by this fungus; in the case of glucoamylase, where the method of fermentation is used to produce that enzyme.
Current Research
"Effect of Ozone on Aspergillus niger Causing Black Rot Disease in Onion"[8]
In vegetation, the Black Rot disease affects the onion, which is caused by Aspergillus niger. A. niger is a growing problem in the storage and transit of onions because an infected product would have growth of a black powdery spore masses of A. niger on the outer skin of the onion. For raw agricultural crops, ozone gas or ozone is added to the water for cleaning the crops. Usage of ozone as a disinfectant has shown to be effective in destroying pathogens and organisms that cause food to spoil; therefore it would be beneficial in extending storage life of foods. Ozone was approved by the FDA in 2002 for use in the food industry as it was titled with (GRAS), “Generally Recognized as Safe” status in the United States.
Samples of isolated A. niger infected onion samples were used and the isolate was placed to be grown in vitro on potato dextrose agar medium at 25 degrees C, for five days. Collection of A. niger spore was carried out through filtration into a suspension, and then transferred to tubes which were stored at 20 C to prevent premature spore germination. Samples of spore suspensions were placed under four different treatments, T1 contained (spore suspension + ozone), T2 (spore suspension + ozone + 0.1% sucrose), T3 control (spore suspension + 0.1% sucrose) and T4 control (spore suspension only). Those samples that faced no exposure to ozone were T3 and T4 controls and experienced incubation period for 6-hour intervals while counts for spore germination were made. A. niger spore suspensions with ozone exposure (T1, T2) were treated similarly to those of T3, T4 except T1 and T2 suspensions were plated on PDA medium to study effects of ozone on the fungal colony morphology. T1, T2 inoculated PDA medium endured a 7 day incubation period.
Results on the effect of ozone on A. niger were that the ozone did not prevent spore germination of A. niger. On the contrary, ozone caused an increase in spore germination which was determined by the lower number of germinated spore in the control T3, T4. In another experiment, the inhibitory effects of ozone on the mycelium of Aspergillus niger was conducted (Chynoweth et al., 1997) [9] and the result was that at ozone concentration of 2.2mg/L, prevention of mycelium growth was successful. Comparison of these two experiments, one of ozone effect on mycelium of A. niger and the other on ozone effect of conidia of A. niger indicates that fungicidal effect of ozone did not affect the processes that cause spore germination. Spore germination in A. niger was not affected by ozone, except changes in colony morphology occurred. Spores treated with ozone and later cultured in vitro on PDA plate, caused change in sporulation; resulted in non-sporulating colonies that could be identified as grey patches in comparison to the surrounding sporulating colonies which were black in color. But, not all spores that were exposed to ozone resulted in unsuccessful sporulation, only spore suspensions that faced increased exposure (at 4.80mg/L) concentration to ozone were unsuccessful in sporulation. Resistant fungal spore can be effected by a pre-treatment of ozone to increase germination, and then with a higher mycelial inhibitory dose of ozone during onion storage. Ozone treatments produced many colonies with sterile mycelium in the next generation which can be controlled efficiently by adding the effective inhibitory ozone dose instead of that of the spore. From these studies, methods were conducted to help management of the black rot disease of the onion by A.niger during storage.[8]
"Regulation of Alternative Oxidase at the Transcription Stage in Aspergillus niger Under the Conditions of Citric Acid Production"[10]
Strain WU-2223L of A. niger, is one that produces citric acid and this strain has a cyanide-insensitive respiratory pathway that is catalyzed by alternative oxidase. Alternative oxidase can be found in many higher plant species, algae and fungi and its regulation under the conditions of citric acid production was deciphered from the transcription level of alternative oxidase gene (aoxl). Analyses of WU-2223L concluded there is only one copy of the aoxl on the chromosome. Alternative oxidase activity encoded by the aoxl gene is regulated at the transcription stage following the conditions that WU-2223L was tested under as well as for production of citric acid. This was determined by measuring alternative oxidase activities and transcription levels of aoxl gene.
An extra accumulation of citric acid by A. niger can be reasoned that the change in metabolism related to functioning of the cyanide=insensitive respiratory pathway catalyzed by alternative oxidase caused the extra citric acid production. Authors of this study hypothesized that the function of the alternative oxidase is essential to the increased production of citric acid by A. niger and that it must be important for the reoxidation of a large amount of NADH that is produced from the process of glycolysis. Results of this study concluded that alternative oxidase activity during the production of citric acid is regulated at the transcription stage. Therefore, A. niger WU-2223L has only one aoxl gene that encodes for an alternative oxidase on its homolog chromosome and to confirm that the contribution of the alternative oxidase is encoded by aoxl gene to production of citric acid can be done so by disrupting the aoxl gene causing an over expression of aoxl, which would result in overproduction of citric acid. [10]
"Recombinant bacterial hemoglobin alters metabolism of Aspergillus niger"[11]
Aspergillus niger is used widely to produce enzymes and organic acids, however there is a drawback to the production when the ongoing issue of an limited supply of oxygen that is required in order for the respiratory metabolism of A. niger in industrial fermentation. The study was focused on the effect on the metabolism of A. niger when VHB is expressed in the cell; to further determine if this method of metabolic engineering could be a resolution to the problem that the fungus has with limited oxygen supply. Results from the study indicated that VHB did have a profound impact on the metabolism of A. niger under conditions where oxygen is limited. When VHB is present, there is less stress on the cells that usually are stressed during short supply of oxygen. Although the process of how VHB is able to fix the problem of limited oxygen are not concise yet, but based on the results obtained from the study, it is evident that VHB interferes with the redox metabolism of the cell and helps resolve the issue. [11]
References
- ↑ Oliveira JM, Van der Veen D, De Graaff, LH, Qin, L. (2008) Efficient Cloning System for Construction of Gene Silencing Vectors in Aspergillus Niger. Applied microbiology and biotechnology. Vol. 80, Issue 5. p. 917-924. DOI 10.1007/s00253-008-1640-x
- ↑ 2.0 2.1 Schuster, E., Dunn-Coleman, N., Frisvad, J., Van Dijck, P. (2002). On the safety of Aspergillus niger – a review. Applied Microbiology and Biotechnology. Vol. 59, Numbers 4-5, pages 426-435. DOI 10.1007/s00253-002-1032-6
- ↑ Andersen MR, Nielsen ML, Nielsen J. (2008) Metabolic model integration of the bibliome, genome, metabolome and reactome of Aspergillus niger. Molecular Systems Biology] 4: 178, pages 1-13. DOI 10.1038/msb.2008.12
- ↑ Semova, N., Storms, R., John, T., Gaudet, P., Ulycznyi P., et al. (2006). Generation, annotation, and analysis of an extensive Aspergillus niger EST collection. BMC Microbiology. v.6:7. DOI 10.1186/1471-2180-6-7
- ↑ 5.0 5.1 5.2 Pel, HJ., de Winde, JH., Archer, DB., Dyer, PS., Hofmann, G., et al. (2007). Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nature Biotechnology, 25. pages 221-231. DOI 10.1038/nbt1282
- ↑ Wadman, M., de Vries, RP., Kalkhove, SIC., Veldink, GA., Vliegenthart JFG. (2009) Characterization of oxylipins and dioxygenase genes in the asexual fungus Aspergillus niger. BMC Microbiology. v.9:59. DOI 10.1186/1471-2180-9-59
- ↑ Deepake, U., (2009) Aero-microbiological studies of Moisture Affected Buildings in the Indoor Environment. Journal of Young Investigators. Vol. 19, Issue 11.
- ↑ 8.0 8.1 8.2 Vijayanandraj, V.R., Nagendra Prasad, D., Mohan, N., Gunasekaran, M. (2006). Effect of Ozone on Aspergillus niger Causing Black Rot Disease in Onion. Ozone: Science & Engineering. Vol. 28, Issue 5. pages 347-350.
- ↑ Chynoweth, D., (1997). ‘‘Influence of Ozone on Growth and Accumulation of Microorganisms in Air Conditioning Systems’’, Proposal for The Canadian Institute for Advanced Research (CIAR), Canad.]
- ↑ 10.0 10.1 Hattori, T., Kino, K., Kirimura, K. (2009). Regulation of Alternative Oxidase at the Transcription Stage in Aspergillus niger Under the Conditions of Citric Acid Production. Vol. 58, Issue 58. pages 321-325.
- ↑ 11.0 11.1 Hofmanna, G., Dianoa, A., Nielsen, J. (2009). Recombinant bacterial hemoglobin alters metabolism of Aspergillus niger. Metabolic Engineering. Vol. 11, Issue 1. pages 8-12. DOI 10.1016/j.ymben.2008.07.002


