Thermus aquaticus: Difference between revisions
imported>Golareh Sina m (→Ecology) |
imported>Golareh Sina |
||
| Line 64: | Line 64: | ||
Recent studies have emphasized the role of disulfide bonds in stabilizing structure of intracellular proteins of Thermus Aquaticus among some other thermophiles. Previously the popular belief was disulfide bonds are only present in extracellular proteins where they stabilize folded proteins against harsh conditions and are rarely found in the cytosil. The specific protein which seems to be responsible for the formation of intracellular disulfide bonds seems to be Protein Disulfide Oxiidoreductase(PDO), which functions as a cytoplasmic PDI. It has been suggested that Eucaryotic enzyme PDI, found in the Endoplasmic Riticulum where it catalyzes isomerization of protein disulfide bonds, has evolved from a protein similar to thermophilic PDO. More research needs to be done on this subject. | Recent studies have emphasized the role of disulfide bonds in stabilizing structure of intracellular proteins of Thermus Aquaticus among some other thermophiles. Previously the popular belief was disulfide bonds are only present in extracellular proteins where they stabilize folded proteins against harsh conditions and are rarely found in the cytosil. The specific protein which seems to be responsible for the formation of intracellular disulfide bonds seems to be Protein Disulfide Oxiidoreductase(PDO), which functions as a cytoplasmic PDI. It has been suggested that Eucaryotic enzyme PDI, found in the Endoplasmic Riticulum where it catalyzes isomerization of protein disulfide bonds, has evolved from a protein similar to thermophilic PDO. More research needs to be done on this subject. | ||
Besides thermophiles, elevated intracellular disulfide bonding has been seen in other extremophiles including; halophiles, alkalophiles, acidophiles, and radiant tolerant organisms. This supports the role of intracellular disulfide bonds in stabilizing proteins in all types of extreme conditions. | Besides thermophiles, elevated intracellular disulfide bonding has been seen in other extremophiles including; halophiles, alkalophiles, acidophiles, and radiant tolerant organisms. This supports the role of intracellular disulfide bonds in stabilizing proteins in all types of extreme conditions. This study sheds some light on different methods used by organisms to stabilize their proteins to adapt to "exotic" environments. | ||
Controversy: After isolation of Thermus Aquaticus, samples of it were deposited in the American Type Cultures collection, a public repository and other scientists had access to them and were able to do more research. By about 1980's , it became obvious that the potential for commercializing the enzymes from this species would prove to be very high and profitable. Hoffman La Roche, a swiss based pharmacuetical company, patent the Taq Polymerase enzyme and the National Parks system were not receiving any of the profits even though the organism was discovered at a national park which is a public property. National Park Services called this "the great Taq ripoff". Since then researchers at the national parks are required to sign an agreement of benefit sharing so a portion of the profits would be returned to the parks. Meanwhile a fight for patent rights of the Taq polymerase is still going on. The European Patent office revoked Hoffman La Roche's patent claiming Taq Polymerase is naturally occurring and finding this enzyme was not a "novel invention". While La Roche is appealing this decision, a Russian scientist named Stanislav Gorodetsky is claiming he and his research group were first to isolate the enzyme and they should somehow be involved in the profit sharing. | |||
==References== | ==References== | ||
Revision as of 21:52, 22 April 2009
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is May 21, 2009. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
| Thermus aquaticus | ||||||
|---|---|---|---|---|---|---|
[[image: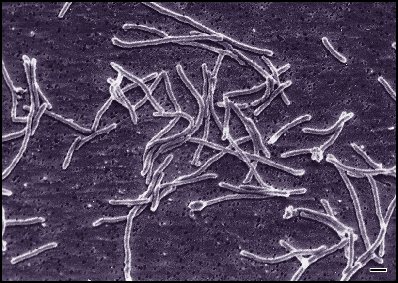 |200px|]] |200px|]] | ||||||
| Scientific classification | ||||||
|
Description and significance
. Thermus Aquaticus isolated in 1969 by brocks and Freeze of University of Indiana. It is a gram negative bacteria both motile (presence of a flagellum) or immotile. Comparisons between structures of Thermus Aquaticus and E. Coli have shown many similarities linking them to a common ancestor. YT-1 gene extracted from Thermus Aquaticus is structurally similar to Rec A from E. Coli homologue and comaparisons between Klentaq1, a large fragment of Taq DNA polymerase and the Klenow fragment of E Coli DNA polymerase reveal identical C terminals and very similar N terminals. Discovery of this species of thermophiles primarily came as a surprise to researchers and very soon proved to be and extremely important tool in many fields of sciences including biology, microbiology, genetics, diagnostics, clinical laboratories, forensic and environmental sciences, hereditary studies and paternity testings. The importance of this discovery comes from the high thermostability of the Thermus Aquaticus proteins. The taq polymerase plays an extremely important role in Polymerase Chain Reaction. PCR is an amplification process of one or a few copies of DNA using thermal cycling introduced by Kary Mullis in 1984 winning him the Noble peace prize in 1993. The enzymes found in the Thermus Aquaticus are able to withstand the heat in the denaturing of the newly formed DNA so strands can seperate and act as templates for the next cycle of PCR. The Taq polymerase with an optimum activity at 72-80 degrees celsius and a half life of 9 minutes at 97.5 degrees celsius can replicate 9000 base pairs in less than 10 seconds, replaced DNA polymerase from E. Coli and presently still plays an extremely important role in gaining insight into realm of biotechnology.
Genome structure
Thermus Aquaticus contains pillus like structures used in conjugation. 12 genes in 3 loci were found to encode for preplin like proteins essential for natural transfomation. It has double stranded circular DNA with a length of 2,338,193 nt. with a replicon type WGS, (Master Wgs), no pseudogenes, 53 structural RNA's, 1982 protein codings, and pTT27 plasmid.
Cell structure and metabolism
Thermus Aquaticus not only can function at high temperatures but they thrive in higher temperatures. Optimum growth is seen between 60 and 75 degrees celsius (could go as low as 35 degrees celsius to as high as 85 degrees celsius). PH ranges from 7.5 to 8.0 generally but some strains could be between 5.1 to 9.5. This organism is a chemotroph using carbohydrates, amino acids, caboxylic acids and peptides for growth. Monosaccharides are generally used for carbons sources but sucrose, maltose(Icelandic stains) and even some fewer strains use glucose. There is also a variance in the proteins isolated from different strains; Elastin, Fibrin and Casein. Not all strains can hydrolize all substrates. Membranes of these proteins are remarkably temperature stable and the heat stability of the enzymes and proteins synthesis systems allow them to function efficiently at high tempratures. Many factors contribute to the stability of the proteins:
1) Highly organized hydrophobic interiors 2) More hydrogen bonds and presence of other non covalent bonds strengthen the structure. 3) Larger quantities of amino acids like Proline make peptide chain less flexible. 4) Proteins aided and stabilized by special chaperone proteins. 5) Some evidence that DNA is stabilized by histone like proteins. 6) Their membrane lipids tend to be more saturated and more branched and posses higher molecular weight resulting in a higher melting point and in turn more thermostable.
Ecology
Thermus Aquaticus was first isolated in the Great Fountain region of Yellowstone National Park from neutral and alkaline springs in 1969 by Brocks and Freeze. This discovery disproved the previous beliefs that bacteria could not function properly at higher temperatures. After this discovery, some strains of Thermus Aquaticus were discovered in hot springs in Iceland and hydrothermal vents in other parts of the world.
Pathology
Thermus Aquaticus has not been associated with any known pathology.
Application to Biotechnology
Enzymes derived from Thermus Aquaticus have had an incredibly important role in facilitating many aspects of biotechnology correlated with DNA amplification and enabling researchers to study proteins and enzymes under conditions not possible before and this is all due to the thermostability of the protiens and there ability to function at even higher rates at high temperatures. Some of the isolated enzymes and their roles in facilitaitng applications in biotechnology are as follows:
1) Adolase- a themostable enzyme (protein enzyme that functions well at high temperature. 2) RNA polymerase- first polymerase isolated from Taq in1974. 3) Restriction Endonucleases 4) DNA polymerase- isolated in 1976, could be isolated in purer form and later discoveered to be used in PCR, for amplifying short segments of DNA (before the discovery of the Taq DNA, enzymes needed to be added after each cycle of denaturing of DNA, but with the use of the Taq DNA polymerase it was not necessary anymore.) One single copy of genomic sequence was amplified by a factor of more than 10 million(One ng of DNA template was amplified up to 35 kb and target DNA molecule present only once in a sample of 105 cells) with improved base pair fidelity and the PCR product used as primers for maximum yield. The specificity , sensitivity , yield and length of product could be amplified. This enzyme was soon cloned, sequenced, and produced in mass quantities for commercial sale. 5) Other enzymes with high optimal temperatures allowing researchers to study them in extreme conditions are:DNA ligase, Alkaline Phosphotase, NADH Oxidase, Isocitrade, Dehydrogenase, Amylomaltase and Fructose1,6-Biophosphate-Dependedent L Lactate Dehydrgenase.
Besides the revolutionary changes in PCR, LCR, using Taq Ligase can amplify genetic sequences of stretches of DNA that posses a desired sequence million or more times within hours. It can amplify and screen in a single step and screen for mutations simultanously. LCR is useful in testing for hereditary diseases, revealing hidden infections and distinguishing between drug resistant and drug sensitive strains of viruses and bacteria .
Current Research
Recent studies have emphasized the role of disulfide bonds in stabilizing structure of intracellular proteins of Thermus Aquaticus among some other thermophiles. Previously the popular belief was disulfide bonds are only present in extracellular proteins where they stabilize folded proteins against harsh conditions and are rarely found in the cytosil. The specific protein which seems to be responsible for the formation of intracellular disulfide bonds seems to be Protein Disulfide Oxiidoreductase(PDO), which functions as a cytoplasmic PDI. It has been suggested that Eucaryotic enzyme PDI, found in the Endoplasmic Riticulum where it catalyzes isomerization of protein disulfide bonds, has evolved from a protein similar to thermophilic PDO. More research needs to be done on this subject.
Besides thermophiles, elevated intracellular disulfide bonding has been seen in other extremophiles including; halophiles, alkalophiles, acidophiles, and radiant tolerant organisms. This supports the role of intracellular disulfide bonds in stabilizing proteins in all types of extreme conditions. This study sheds some light on different methods used by organisms to stabilize their proteins to adapt to "exotic" environments.
Controversy: After isolation of Thermus Aquaticus, samples of it were deposited in the American Type Cultures collection, a public repository and other scientists had access to them and were able to do more research. By about 1980's , it became obvious that the potential for commercializing the enzymes from this species would prove to be very high and profitable. Hoffman La Roche, a swiss based pharmacuetical company, patent the Taq Polymerase enzyme and the National Parks system were not receiving any of the profits even though the organism was discovered at a national park which is a public property. National Park Services called this "the great Taq ripoff". Since then researchers at the national parks are required to sign an agreement of benefit sharing so a portion of the profits would be returned to the parks. Meanwhile a fight for patent rights of the Taq polymerase is still going on. The European Patent office revoked Hoffman La Roche's patent claiming Taq Polymerase is naturally occurring and finding this enzyme was not a "novel invention". While La Roche is appealing this decision, a Russian scientist named Stanislav Gorodetsky is claiming he and his research group were first to isolate the enzyme and they should somehow be involved in the profit sharing.
References
Barnes,W.M. PCR amplification of up to 35 kb DNA with high fidelity and high yield from bacteriophage templates-proceedings of the National Academy of Sciences of the United States of America v.91(March 15,1994)p.2216-20
Weiss, R. Hot prospect for new gene amplifier-Science v.254(November 29,1991)p.1292-3
Saiki, R.K.,et.al., Primer directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science v.239 (January 29,1988)p.487-91.
<http://microbewiki.kenyon.edu/index.php/Thermus> The Genomics of Disulfide Bonding and Protein Stabilization in Thermophiles Beeby M, O'Connor BD, Ryttersgaard C, Boutz DR, Perry LJ, et al. PLoS Biology Vol. 3, No. 9, e309 doi:10.1371/journal.pbio.0030309
Dalton, R. Patent ruling could cut PCR enzyme prices. Nature v. 411 no. 6838 (June 7 2001) p. 622
Dickson, D. European patent for PCR enzyme clouded by Russian claim. Nature v. 364 (July 1 1993) p. 2