Food reward: Difference between revisions
imported>Gareth Leng |
imported>Gareth Leng |
||
| Line 67: | Line 67: | ||
==='''The effect of hormones on the dopaminergic reward system'''=== | ==='''The effect of hormones on the dopaminergic reward system'''=== | ||
The role of hormones such as [[leptin]], [[ghrelin]] and [[insulin]] in the homeostatic control of energy balance has been extensively studied. Levels of leptin and insulin reflect the size of energy stores, such as [[adipose tissue]], and feed into the medial hypothalamus to influence feeding behaviours and regulate food intake and energy expenditure in response to metabolic demand | The role of hormones such as [[leptin]], [[ghrelin]] and [[insulin]] in the homeostatic control of energy balance has been extensively studied. Levels of leptin and insulin reflect the size of energy stores, such as [[adipose tissue]], and feed into the medial hypothalamus to influence feeding behaviours and regulate food intake and energy expenditure in response to metabolic demand. Recent evidence also implicates a role for such hormones in dopamine reward pathways [[#References|Palmiter R.D (2007)]], [[#References|(Figlewicz DP, Benoit SC, 2009)]]. | ||
Receptors for these hormones are located on dopamine neurones in the | Receptors for these hormones are located on dopamine neurones in the VTA [[#References|(Magni P. et al, 2009)]], and ligand binding results in activation e.g. by ghrelin [[#References|(Abizaid A ''et al.'' 2006)]], or inhibition e.g. by insulin or leptin of dopamine signalling to the nucleus accumbens (NAc) [[#References|(Magni P et al, 2009)]]. These alterations in dopamine signalling pathways have complex effects on eating behaviours. Many studies have reinforced the hypothesis that insulin and leptin attenuate the food reward, reducing incentive to eat [[#References|(Figlewicz DP, Benoit SC (2009)]]. For example, behavioural studies showed that administering insulin or leptin to rats affected conditional place preference (CPP), which assesses the ability to relate a particular food reward to a particular environment [[#References|(Figlewicz DP, 2003)]]. Rats were fed a high fat diet and underwent a ‘training period’ prior to the test involving intracerebroventricular administration of insulin or leptin. CPP was only abolished in those rats that received insulin or leptin treatment before or during the test as well as in training, whilst those who only received it in training maintained a normal CPP [[#References|(Figlewicz, D.P. et al, 2004)]]. This suggests that insulin and leptin influence the retrieval of food reward associations rather than the initial formation of these associations; presumably as a consequence of inhibition of dopamine reward pathways. These results have been reinforced by studies which have shown decreased sucrose self-administration in response to insulin or leptin administration and decreased sucrose licking following insulin treatment [[#References|(Sipols AJ et al, 2000)]]. The high levels of insulin and leptin associated with obesity impair dopamine food reward pathways resulting in abnormal eating behaviours [[#References|(Figlewicz DP, Benoit SC, 2009)]]. | ||
''Table 1: Summary of effects of centrally administered insulin and leptin on reward behaviours'' | ''Table 1: Summary of effects of centrally administered insulin and leptin on reward behaviours'' | ||
| Line 108: | Line 108: | ||
*Adapted from [[#References|(Figlewicz, D.P. et al, 2004)]] | *Adapted from [[#References|(Figlewicz, D.P. et al, 2004)]] | ||
Ghrelin can increase dopamine signalling via ghrelin receptors on VTA neurones, via direct activation and also indirect manipulation of inputs onto the VTA to those of an excitatory nature | Ghrelin can increase dopamine signalling via ghrelin receptors on VTA neurones, via direct activation and also indirect manipulation of inputs onto the VTA to those of an excitatory nature. However, it remains unclear whether this is a significant part the mechanism by which ghrelin stimulates feeding [[#References|(Palmiter R, 2007)]]. | ||
A major difficulty in elucidating the | A major difficulty in elucidating the roles of these hormones in reward systems and in regulation of body weight, is that, due to the involvement of multiple hormones, manipulation of one results in activation of compensatory mechanisms, masking the physiological role of the manipulated hormone. Furthermore, by manipulating levels of these hormones to abnormal levels, we can suggest potential functions for them, but this may not be relevant when they are found at physiological concentrations. It should also be noted that the VTA possesses other neurons such as GABA-projection neurons, which also express receptors for the discussed hormones. Therefore, we can not assume that these hormones affect feeding behaviours solely by their action on dopamine reward pathways. It is also unclear whether these hormones act directly on dopamine reward pathways; insulin and leptin may influence dopamine reward systems by altering the activity of secondary peptide effector pathways, such as orexin A and melanocortins [[#References|(Figlewicz DP, Benoit SC, 2009)]]. | ||
Much work, therefore, remains to be done to decipher the significance of results from these studies and the specific roles of hormones in food reward pathways | Much work, therefore, remains to be done to decipher the significance of results from these studies and the specific roles of hormones in food reward pathways. | ||
==='''Opioid and cannabinoid systems'''=== | ==='''Opioid and cannabinoid systems'''=== | ||
Revision as of 13:19, 14 March 2010
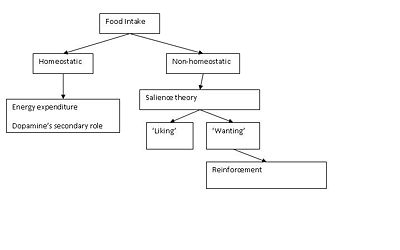
Food intake is driven by both 'homeostatic feeding' (energy demands) and ‘non-homeostatic feeding’ (pleasure associated eating or preferred food); the latter is associated with food reward, which involves both 'liking’ (pleasure/palatability) and ‘wanting’ (incentive motivation) according to the salience theory. Experiments in mice suggest that ‘liking’ involves the release of mu-opioids in the nucleus accumbens, ventral pallidum, parabrachial nucleus, and nucleus of the solitary tract, while mechanisms attributed to ‘wanting’ implicate the neurotransmitter dopamine released in brain areas such as the prefrontal cortex (PFC), amygdala, hypothalamus, and projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc)[1].
Dopamine has an important role in both energizing and reinforcing feeding. The focus of this article is the reinforcement of feeding, which is the primary role of dopamine and its involvement in food reward pathways. The action of dopamine in the dopaminergic systems and the interaction of these systems with other reward systems is described. Finally the effect of other hormones such as insulin, ghrelin, and leptin, on food reward is studied through their association with dopamine. The final part of this article links these processes, particularly the dopaminergic pathway, with obesity. A description of what can go wrong, changes in the distribution of dopamine receptors, and a genetic approach is given, linking the function of the dopaminergic system and obesity by a gene, related to the reinforcing properties of food behaviour.
| Key Definitions |
|---|
| Wanting/ Incentive salience - The motivational aspect of a stimulus that transforms the sensory information into a more desirable stimulus. |
| Liking - Immediate pleasure from consumption |
| Hedonia - The feeling of pleasure |
| Motivation- The direction towards a particular behaviour to achieve a goal |
| Reinforcement - The process by which a stimulus strengthens a behavioural response so that the probability of response is increased when the stimulus is presented again. |
Motivated behaviour and food as a reinforcer
The underlying pathways in motivating feeding behaviour are far more complex than a simple homeostatic system, responding to metabolic and satiety signals from the gut. One possibility is that the brain’s reward systems react to stimuli such as sight, smell and taste, or other cues that predict food. However, hunger cannot result in unconditioned goal-directed behaviour [2]. Chance encounters with various palatable foods are required before goal-directed behaviour can occur, which links the internal needs with the salience of environmental stimuli [3]
For example, an infant recognises[4]and learns to seek out (Johanson & Hall 1979)sweet tastants, but the desire for a particular food is controlled by the interaction of peptide levels with the brain circuitry. Where the peptide levels are related to hunger, and the brain circuitry coding the animal’s reinforcement history for that specific food. Subsequently, the infant will indiscriminately taste both food and non-food objects, until it has received reinforcing feedback from sufficient stimuli (Wise, 2006). In addition, the monkey’s appetite for a yellow banana requires the prior learning of the relation of the sight of the yellow skin of a banana, with the sweet taste of the white banana meat (Wise, 2004b) plus the consequences resulting from the ingestion of the fruit. Therefore, preference for a specific food, results only when the post-ingestional consequences of that food’ reinforce’ the tendency to eat that food. For the above reasons, food is considered to be a strong reinforcer. Moreover, when the response of a behaviour stimulated by a reinforcer increases the rate of that specific behaviour; that is known as positive reinforcement or reward learning, and the positive events are called rewards (Epstein, 2007). The reinforcing efficacy of food reward is the ability of the reward to maintain rather than to establish behaviour; consequently the stimulus learning contributes to the response learning.
The first evidence for the involvement of dopamine in food reward came from studies in rats, where dopamine antagonists blocked the rewarding effects of brain stimulation (Liebman & Butcher 1974; Fouriezos & Wise 1976) and of psychomotor stimulants.
Food reward pathways
The role of the Mesolimbic Dopaminergic Reward System
In the ‘reward circuit’, projections from the Ventral Tegmental Area (VTA) to the Nucleus Accumbens (NAc) have received the most attention due to the focus of studies on the hedonic impact from drugs and their possible roles in reinforcement, reward and addiction. These results have often led to the conclusion that dopamine action in the NAc is needed for motivation to acquire food or addictive drugs. Most reviews suggest that the projections from the VTA-NAc are needed for the motivation to eat but not for the food consumption. Lesion experiments have shown that even when the VTA-NAc pathway has been destroyed the mice still manage to eat. (Wise, 2006)
The Dopamine Hypothesis
Dopamine signalling from the VTA to the NAc, hippocampus, amygdala and/or pre-frontal cortex promotes reward-related activities. Dopamine signalling in these brain regions focuses attention to salient environmental events and thereby facilitates behaviour towards directed goals. Also it is thought that dopamine released from the VTA also forms associations to promote learning between food reward and the environment (Palmiter 2007)
However the role of mesolimbic dopamine seems to be controversial. Dopamines’ (DA) possible role in relation to reward?
• Hedonia – Dopamine in the NAc acts as a pleasure neurotransmitter. Proposed due to drug activity. Not all rewards activate the reward system suggesting that the mesolimbic pathway is not solely hedonic.
• Learning – predictions of future rewards, NAc and VTa lesions do not affect this part but lack the motivation for the reward.
• Incentive Salience – the ‘wanting’ of the reward, released when there is a stimulus worth working hard for. In absence of DA the environmental stimulus goes unnoticed and the animal eventually dies due to starvation and dehydration.
The incentive salience theory seems to best fit the data in this field according to (Berridge (2007). Therefore dopamine causes the wanting of the reward after the appropriate stimuli have been processed in the reward system. An elevation of dopaminergic transmission is needed to form these associations. It has been shown that an increase in extracellular dopamine is seen in regard to natural rewards, food, water and sex, during acute administration (Wise & Rompre 1989, Spanagel & Weiss 1999). However it must be noted that novelty is an important factor in the increased release from the NAc.
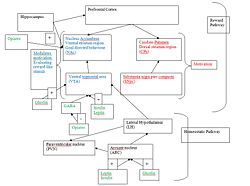
It has been suggested by (Palmiter, 2008 that the role of dopamine in motivation is split between the 2 dopaminergic pathways; the NAc and CPu pathways( see diagram). The SNpc-CPu pathway is essential for motivation with dopamine signalling from the VTA-NAc needed in regard to modulating the actions of the other dopaminergic pathway.
The substantia nigra pars compacta (SNpc) to the caudate putamen (CPu): A critical dopaminergic pathway
It is believed that the midbrain dopamine neurons are the key neural components for reward mechanisms. Creation and observation of dopamine deficient (DD) mice implied that DD mice starve because they are not motivated to respond to hunger signals. Thus, its been proposed that dopamine is crucial for mice to engage in the majority of goal-directed or motivated behaviours.
However there is much controversy as to the pathway used; a universal finding is the involvement of the striatum, the input structure of the basal ganglia in a circuit responsible for mediating goal-directed behaviour, with the striatum’s central role being the processing of reward like stimuli (Delgado, MR. (2007)). The two proposed pathways are from the ventral tegmental area (VTA) to nucleus accumbens (NAc) (ventral striatum); or the substantia nigra pars compacta (SNpc) to the caudate putamen (CPu) (dorsal striatum)
The striatum includes not only the dorsal region, which encompasses the caudate nucleus and putamen, but also the ventral region that includes the core and shell of the nucleus accumbens, see figure 2 (Wickens, JR. et al (2007)).
Our understanding of how reward information is processed comes mailly from studies in animal models. One study, using nonhuman primates, found that striatal neurons responded to the anticipation and delivery of reward (Delgado, MR. (2007)). Another found a reward-related dopamine response specifically in the mouse dorsal striatum, correlated with the delivery of food reward (Natori S et al. (2009)). The importance of the dopamine system in the dorsal striatum is demonstrated in a study using DD mice whose dopamine signalling is restored by viral rescue (Palmiter RD (2008), (Darvas M, Palmiter RD(2009)) . These mice learned to lever press for food rewards as quickly as control mice and their motivation to work for food was restored (Darvas M, Palmiter RD (2009)). An important finding was that in DD deficient mice feeding was never restored after viral transduction in the NAc (Palmiter RD (2008)).
Recently, advances in neuroimaging techniques has allowed researchers to extend such investigations to the human brain. Dopamine release increases in dorsal striatum of hungry participants when stimulated with food items, demonstrating its involvement in reward processing. During the delivery of rewards, fMRI signals were higher in the dorsal striatum, particularly the head of the CPu (Delgado MR (2007)). These findings strongly suggest the human dorsal striatums involvement in reward processing; with the CPu being an integral structure of a circuit involved in learning and updating current rewards with the aim of maximizing reward consumption.
The role of dopamine signalling in the CPu cannot be ignored as viral restoration rescued feeding, whereas in the NAc it did not. It has been proposed that dopamine signaling in the CPu is essential for motivation while dopamine signaling in the NAc modulates this motivation and evaluation of reward like stimuli, see figure 2 (Palmiter RD (2008)).
The effect of hormones on the dopaminergic reward system
The role of hormones such as leptin, ghrelin and insulin in the homeostatic control of energy balance has been extensively studied. Levels of leptin and insulin reflect the size of energy stores, such as adipose tissue, and feed into the medial hypothalamus to influence feeding behaviours and regulate food intake and energy expenditure in response to metabolic demand. Recent evidence also implicates a role for such hormones in dopamine reward pathways Palmiter R.D (2007), (Figlewicz DP, Benoit SC, 2009).
Receptors for these hormones are located on dopamine neurones in the VTA (Magni P. et al, 2009), and ligand binding results in activation e.g. by ghrelin (Abizaid A et al. 2006), or inhibition e.g. by insulin or leptin of dopamine signalling to the nucleus accumbens (NAc) (Magni P et al, 2009). These alterations in dopamine signalling pathways have complex effects on eating behaviours. Many studies have reinforced the hypothesis that insulin and leptin attenuate the food reward, reducing incentive to eat (Figlewicz DP, Benoit SC (2009). For example, behavioural studies showed that administering insulin or leptin to rats affected conditional place preference (CPP), which assesses the ability to relate a particular food reward to a particular environment (Figlewicz DP, 2003). Rats were fed a high fat diet and underwent a ‘training period’ prior to the test involving intracerebroventricular administration of insulin or leptin. CPP was only abolished in those rats that received insulin or leptin treatment before or during the test as well as in training, whilst those who only received it in training maintained a normal CPP (Figlewicz, D.P. et al, 2004). This suggests that insulin and leptin influence the retrieval of food reward associations rather than the initial formation of these associations; presumably as a consequence of inhibition of dopamine reward pathways. These results have been reinforced by studies which have shown decreased sucrose self-administration in response to insulin or leptin administration and decreased sucrose licking following insulin treatment (Sipols AJ et al, 2000). The high levels of insulin and leptin associated with obesity impair dopamine food reward pathways resulting in abnormal eating behaviours (Figlewicz DP, Benoit SC, 2009).
Table 1: Summary of effects of centrally administered insulin and leptin on reward behaviours
| Behaviour | Effect of insulin | Effect of leptin |
|---|---|---|
| Brain self-stimulation | Decrease | Decrease |
| Relapse to heroin suckling | Not tested | Decrease |
| Acute sucrose suckling | Decrease | Not tested |
| Food-condtioned place preference | Decrease | Decrease |
| Sucrose self-administration | Decrease | Decrease |
| Acute chow intake | Decrease | Decrease |
| Opioid-stimulated sucrose | Decrease | Decrease |
- Adapted from (Figlewicz, D.P. et al, 2004)
Ghrelin can increase dopamine signalling via ghrelin receptors on VTA neurones, via direct activation and also indirect manipulation of inputs onto the VTA to those of an excitatory nature. However, it remains unclear whether this is a significant part the mechanism by which ghrelin stimulates feeding (Palmiter R, 2007).
A major difficulty in elucidating the roles of these hormones in reward systems and in regulation of body weight, is that, due to the involvement of multiple hormones, manipulation of one results in activation of compensatory mechanisms, masking the physiological role of the manipulated hormone. Furthermore, by manipulating levels of these hormones to abnormal levels, we can suggest potential functions for them, but this may not be relevant when they are found at physiological concentrations. It should also be noted that the VTA possesses other neurons such as GABA-projection neurons, which also express receptors for the discussed hormones. Therefore, we can not assume that these hormones affect feeding behaviours solely by their action on dopamine reward pathways. It is also unclear whether these hormones act directly on dopamine reward pathways; insulin and leptin may influence dopamine reward systems by altering the activity of secondary peptide effector pathways, such as orexin A and melanocortins (Figlewicz DP, Benoit SC, 2009).
Much work, therefore, remains to be done to decipher the significance of results from these studies and the specific roles of hormones in food reward pathways.
Opioid and cannabinoid systems
Other reward systems, including the endogenous opiate and endocannabinoid systems, also play a role in reward behaviours and interact with dopamine reward pathways (Palmiter R, 2007). Opiates act in the nucleus accumbens to increase ‘wanting’ and ‘liking’ of food rewards (Pecina S, 2008). Opioids also influence mesolimibic dopamine pathways by inhibiting GABAnergic input onto dopamine neurones on the VTA, resulting in increased dopamine release (Spanagel and Weiss, 1999). The endocannabinoid system has also been implicated in reward behaviours, with cannabinoid receptors being expressed in those brain areas implicated in reward, including the mesolimibic system (Solinas M 2008). Endocannabinoids modulate neurotransmission in several brain areas (Maldonado, R et al 2006). For example, cannabinoid receptor agonists stimulate dopaminergic transmission, increasing the reward experienced in response to food and drug abuse (Solinas M 2008). Conversly, antagonising these receptors inhibits activation of dopaminergic transmission by such rewards (Solinas M 2008). The normal physiological effect of the endocannabinoid system on reward systems remains a topic of much dispute, and it has been hypothesised to be involved in the ‘fine-tuning’ of dopaminergic transmission (Solinas M 2008). In summary, the endocannabinoid system has a key role in modulating the many reward pathways.
Food reward and Obesity
The relationship between obesity and the dopaminergic system
There is a difference in dopaminergic activity between obese women in comparison to lean women/men in response to food and satiety. It has been shown that the obese have a higher metabolic activity in the parietal somatosensory area of the cortex which is linked to the sensory mouth, lips and tongue. An increased amount of sensory processing in this brain region could increase the reinforcing properties of food (Epstein 2007).
Deficiencies of the D2 receptor have been suggested to increase the likelihood of being obese. (Wang et al 2001) showed that obese people have fewer D2 receptors in the striatum and with both the D1 and D2 receptors acting synergistically to decrease feeding, this altered expression causes an increased amount of eating. The DRD2 gene is responsible for the reinforcing properties of food/addictive behaviour (Noble 2003). Those who have the allelic variant A1 in this gene have fewer D2 receptors and therefore a decreased amount of dopamine signalling within the brain. This has been shown to be higher in obese individuals making the dopamine reward circuits less sensitive. This could possibly explain why obese people possibly overeat in order to compensate for their lack of reward.
References
- ↑ Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431
- ↑ Changizi MA et al. (2002) Evidence that appetitive responses for dehydration and food-deprivation are learned. Physiol Behav 75:295–304.
- ↑ Wise RA (2006) Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci 361:1149–58
- ↑ Steiner JE et al.(2001). Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev 25:53–74
Abizaid, A. et al. (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 116, 3229–3239
Darvas, M. & Palmiter, RD.(2009) Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviours PNAS 106;34, 14664–14669
Delgado, MR. (2007) Reward-Related Responses in the Human Striatum Ann. N.Y. Acad. Sci. 1104: 70–88
de Wit, H. & Wise, R. A. 1977 Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can. J. Psychol. 31, 195–203.
Epstein LH, Leddy JJ, Temple JL, Faith MS (2007). Food reinforcement and eating: a multilevel analysis. Psychol Bull;133:884–906
Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 284(4):R882-92, 2003 Apr.
Figlewicz DP, Benoit SC. (2009) Insulin, leptin, and food reward: update 2008.Am J Physiol Regul Integr Comp Physiol.296(1):R9-R19
Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats.Physiol Behav 89: 611–616, 2006.
Figlewicz, D.P. et al. (2004). Intraventricular insulin and leptin reverse place preference conditioned with high fat food. Behav. Neurosci. 118, 479–48
Fouriezos, G. & Wise, R. A. (1976). Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res. 103, 377–380.
Johanson, I. B. & Hall, W. G. (1979). Appetitive learning in 1-day-old rat pups. Science 205, 419–421.
Liebman, J. M. & Butcher, L. L. (1974). Comparative involvement of dopamine and noradrenaline in rate-free self-stimulation in substantia nigra, lateral hypothalamus, and mesencephalic central gray. N-S. Arch. Pharmacol.
Magni P. et al. (2009) Feeding behavior in mammals including humans. Ann.N.Y.Acad.Sci. 1163:221-232
Maldonado, R et al (2006). Involvement of the endocannabinoid system in drug addiction. Trends Neurosci; 29, 225-232.
Natori, S. Yoshimi K. Takahashi T. Kagohashi M. Oyama G. Shimo Y. Hattori N. Kitazawa S. (2009) Subsecond reward-related dopamine release in the mouse dorsal striatum . Neuroscience Research 63, 267–272
Noble EP, Noble RE, Ritchie T, Syndulko K, Bohlman MC, Noble LA, et al. D2 dopamine receptor gene and obesity. International Journal of Eating Disorders 1994;15:205–217.
Palmiter R.D (2007) Is dopamine a physiologically relevant mediator of feeding behaviour? TINS 30. 8:375-381
Palmiter, RD. (2008) Dopamine Signaling in the Dorsal Striatum Is Essential for Motivated Behaviors: Lessons from Dopamine-deficient Mice Ann N Y Acad Sci. 1129: 35–46.
Pecina s. Opioid reward 'liking' and 'wanting' in the nucleus accumbens. Physiology & Behavior. 94(5):675-80, 2008 Aug 6.
Spanagel R & Weiss F (1999). The dopamine hypothesis of reward: past and current status. TINS. 22.11: 521-527
Satoh, T. Nakai, S. Sato T., Kimura M. (2003) Correlated Coding of Motivation and Outcome of Decision by dopamine neurons. The Journal of Neuroscience, 23(30):9913–9923
Sipols AJ, Stuber GD, Klein SN, Higgins MS, Figlewicz DP. Insulin and raclopride combine to decrease short-term intake of sucrose solutions. Peptides 21: 1361–1367, 2000.
Solinas M (2008) 'The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008 May;154(2):369-83.
Spanagel R and Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. (1999) 22, 521-527.
Wang et al (2001) Brain dopamine and obesity. The Lancet • Vol 357, 354-357
Wickens, JR. Budd, CS. Hyland, BI. Arbuthnott, GW. (2007) Striatal Contributions to Reward and Decision Making Ann. N.Y. Acad. Sci. 1104: 192–212
Wise, R. A. (2004b). Drive, incentive, and reinforcement: the antecedents and consequences of motivation. Nebr. Symp. Motiv. 50, 159–195.
Wise RA & Rompre PP (1989). Annual review Psychology. 40: 191-225