Reid vapor pressure: Difference between revisions
imported>Milton Beychok m (Added explanation of how the RVP test is performed.) |
imported>Milton Beychok (Added a reference) |
||
| Line 3: | Line 3: | ||
'''Reid vapor pressure''' (RVP), determined by the [[ASTM]] test method D323,<ref>Test method D323-08 of the [[American Society for Testing and Materials]] (ASTM). [http://www.astm.org/ABOUT/aboutASTM.html ASTM website]</ref> is widely used in the petroleum industry to measure the volatility of [[petroleum crude oil]], [[gasoline]] and other petroleum products. It is a quick and simple method of determining the [[vapor pressure]] at 37.8 °[[Celsius|C]] (100 °[[Fahrenheit and Rankine temperatures|F]]) of crude oil and petroleum products having an initial [[boiling point]] above 0 °C (32 °F). | '''Reid vapor pressure''' (RVP), determined by the [[ASTM]] test method D323,<ref>Test method D323-08 of the [[American Society for Testing and Materials]] (ASTM). [http://www.astm.org/ABOUT/aboutASTM.html ASTM website]</ref> is widely used in the petroleum industry to measure the volatility of [[petroleum crude oil]], [[gasoline]] and other petroleum products. It is a quick and simple method of determining the [[vapor pressure]] at 37.8 °[[Celsius|C]] (100 °[[Fahrenheit and Rankine temperatures|F]]) of crude oil and petroleum products having an initial [[boiling point]] above 0 °C (32 °F). | ||
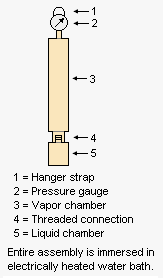
The adjacent drawing depicts the apparatus used for measuring the Reid vapor pressure of gasolines and other products having a vapor pressure below atmospheric pressure. The liquid chamber is filled with chilled liquid sample and the the liquid chamber is then | The adjacent drawing depicts the apparatus used for measuring the Reid vapor pressure of gasolines and other products having a vapor pressure below atmospheric pressure. The liquid chamber is filled with a chilled liquid sample and the the liquid chamber is then quickly connected to the vapor chamber fitted with pressure gauge. The entire asembly is then hung by the hanger strap so that the assembly is immersed in an electrically heated water bath. After 5 minutes, the assembly is removed, shaken and the pressure gauge is read. The assembly is then re-immersed and after another 2 minutes, it is removed, shaken and the pressure gauge is read again. This procedure is repeated until two successive readings are the same.<ref name=Liptak>{{cite book|author=Béla G. Lipták (Editor)|title=Instrument Engineers' Handbook: Process Measurement and Analysis|edition=4th Edition|publisher=CRC Press|year=2003|id=ISBN 0-8493-1083-0}}</ref> | ||
== Importance and uses == | == Importance and uses == | ||
Revision as of 17:42, 31 October 2009
Reid vapor pressure (RVP), determined by the ASTM test method D323,[1] is widely used in the petroleum industry to measure the volatility of petroleum crude oil, gasoline and other petroleum products. It is a quick and simple method of determining the vapor pressure at 37.8 °C (100 °F) of crude oil and petroleum products having an initial boiling point above 0 °C (32 °F).
The adjacent drawing depicts the apparatus used for measuring the Reid vapor pressure of gasolines and other products having a vapor pressure below atmospheric pressure. The liquid chamber is filled with a chilled liquid sample and the the liquid chamber is then quickly connected to the vapor chamber fitted with pressure gauge. The entire asembly is then hung by the hanger strap so that the assembly is immersed in an electrically heated water bath. After 5 minutes, the assembly is removed, shaken and the pressure gauge is read. The assembly is then re-immersed and after another 2 minutes, it is removed, shaken and the pressure gauge is read again. This procedure is repeated until two successive readings are the same.[2]
Importance and uses
Vapor pressure is an important physical property of volatile liquids. It is of critical importance for automotive and aviation gasolines since it affect the starting and warm-up of spark-ignited internal combustion engines as well as the tendency to cause "vapor lock" in the fuel pumps with high operational temperatures and/or high altitudes.
Air pollution regulatory authorities mandate maximum gasoline vapor pressures in many areas so as to limit the evaporative emissions of smog-forming hydrocarbons from gasoline.
Vapor pressure is also important as an indirect measure of the evaporation tendency of volatile petroleum solvents.
The difference between RVP and TVP
True vapor pressure is referred to in the petroleum industry as TVP. Both the Reid vapor pressure (RVP) and the true vapor pressure (TVP) are absolute pressures as distinguished from gauge pressures (see Pressure).
Because of the presence air (and its of water vapor content) in the vapor space within the test method's sample container, as well as some small amount of sample vaporization during the warming of the sample to 37.8 °C (100 °F), the RVP differs by a small amount from the TVP of the sample.
RVP values may be converted to TVP values using the nomograms or the equations provided in Chapter 7 of the AP 42 Compilation of Air Pollutant Emission Factors published by the U.S. Environmental Protection Agency and available online.[3]
Other test methods for certain petroleum products
Test method ASTM D323 is not applicable for liquified petroleum gases (LPG). ASTM D1267 or D6897 should be used for LPG.
ASTM D323 is also not applicable for gasolines containing oxygenated compounds other than methyl tertiary-butyl ether (MTBE).[4] For gasolines containing other oxygenated compounds, refer to ASTM D4953.
References
- ↑ Test method D323-08 of the American Society for Testing and Materials (ASTM). ASTM website
- ↑ Béla G. Lipták (Editor) (2003). Instrument Engineers' Handbook: Process Measurement and Analysis, 4th Edition. CRC Press. ISBN 0-8493-1083-0.
- ↑ AP42, Chapter 7, Section 7.1 On pdf pages 54-56 of 123 pdf pages. All units are U.S. customary units.
- ↑ For more information about MTBE, see Gasoline.