Metabolism/Citable Version: Difference between revisions
imported>David Tribe |
imported>Gareth Leng (first pass copy edit) |
||
| Line 1: | Line 1: | ||
'''Metabolism''' (from [[Greek language|Greek]] ''μεταβολισμός'' "metabolismos") is the [[Biochemistry|biochemical]] modification of [[chemical compound]]s in [[life|living]] [[organism]]s and [[cell (biology)|cell]]s. In common | '''Metabolism''' (from [[Greek language|Greek]] ''μεταβολισμός'' "metabolismos") is the [[Biochemistry|biochemical]] modification of [[chemical compound]]s in [[life|living]] [[organism]]s and [[cell (biology)|cell]]s. In common use, the word is often used to refer to the [[basal metabolic rate]], the "set point" that each person has in breaking down food energy and building up their own body. Sometimes, in [[multicellular]] creatures like humans, it is also used to refer to the overall ingestion of [[food]] and excretion of wastes, and the building up of [[muscles]] and the [[growth]] of the body. This article describes the actual biology of metabolism at a cellular level, which explains just how those processes are carried out. In human and whole animal terms, metabolism also includes the chemical conversion of specific items other than food that may be ingested, like drugs and [[poisons]]. | ||
Metabolism | Metabolism involves: (1) [[anabolism]], in which a cell uses energy and chemical reducing power to ''construct'' complex molecules, and perform such life functions as ''creating'' cellular structure; and (2) [[catabolism]], in which a cell ''breaks down'' complex molecules to yield energy and chemical reducing power. Cell metabolism involves complex sequences of controlled chemical reactions called [[metabolic pathway]]s. Just as the word metabolism can be used to describe processes in a whole organism, such as a person, the terms "anabolism" and "catabolism" can also be used in this way. For example, anabolic processes include building up muscle and adding body weight, and catabolic processes include loss of muscle mass and body [[fat]]. | ||
== History == | == History == | ||
[[Image:SantoriosMeal.jpg|thumb|right|150px|[[Santorio Santorio]] (1561-1636) in his steelyard balance, from <i>Ars de statica medecina</i>, first published 1614.]] | [[Image:SantoriosMeal.jpg|thumb|right|150px|[[Santorio Santorio]] (1561-1636) in his steelyard balance, from <i>Ars de statica medecina</i>, first published 1614.]] | ||
The first controlled experiments | The first controlled experiments on human metabolism were published by [[Santorio Santorio]] in [[1614]] in his book <i>Ars de statica medecina</i>, that made him famous throughout [[Europe]]. Santorio described his long series of personal experiments: in which he weighed himself in a chair suspended from a steelyard balance (see image), before and after eating, sleeping, working, sex, fasting, depriving from drinking, and excreting. He found that by far the greatest part of the food he took in was lost from the body through ''<i>perspiratio insensibilis</i>'' (insensible perspiration). In [[medicine]] and the [[health sciences]] today, "insensible losses" is still used to refer to the amount of fluid lost from the body through perspiration and processes that (unlike urine or feces) do not yield any obvious portion that can be weighed or measured. | ||
<br>At | <br>At about the same time [[Jan Baptist van Helmont]] made the first observations regarding [[photosynthesis]], when he discovered that plant growth required (almost) no soil nutrients. In the 18th century, [[Joseph Priestley|Priestley]] concluded that green plants use CO<sub>2</sub> and release O<sub>2</sub>. In 1804, [[Nicolas-Théodore de Saussure|Nicolas de Saussure]] discovered that the increase in carbon content of plants (i.e. plant growth) arises from the fixation of atmospheric CO<sub>2</sub>.Between 1854 and 1864, [[Louis Pasteur]] discovered that glucose [[fermentation]] is due to [[microorganisms]], and, in 1897, [[Eduard Buchner]] proved that ''cell-free'' yeast extracts could also perform these reactions, and so the ability to ferment was not limited to entire living creatures (cells)- but included certain portions of their physical contents. Subsequent investigations showed that (with a few exceptions) all living organisms metabolize glucose using the [[Glycolysis|same mechanism]]. | ||
== Overview == | == Overview == | ||
[[Image: Metabolism scheme.GIF|thumb|350px|A few of the catabolic pathways in a cell. | [[Image: Metabolism scheme.GIF|thumb|350px|A few of the catabolic pathways in a cell. [[Protein]]s are broken down into [[amino acids]], and fats into glycerol and [[fatty acids]]. [[Carbohydrates]] (mostly sugars and starch) are hydrolyzed into monosacharides like glucose. The [[mitochondrium]] (in green) contains the enzymes that catalyze the [[citric acid cycle]] and [[β-oxidation|beta-oxidation]], as well as the [[electron transport chain]] (where respiration occurs). [[ATP]] is a high-energy molecule. See text for details]] | ||
[[Enzymes]] present in [[cell]]s | [[Enzymes]] present in [[cell]]s can catalyze a large variety of chemical reactions with exquisite specificity. Often, the chemical reactions needed to synthesize useful cell components involve a positive change in [[Gibbs free energy|free energy]], i.e. they are not spontaneous. In these cases, enzymes may couple the non-spontaneous reaction to a second, very spontaneous, reaction, so that the overall process is spontaneous. The spontaneous reaction usually used to drive metabolism is hydrolysis of [[ATP]] in [[ADP]] and [[phosphate]] anion. Therefore, ATP synthesis from ADP and phosphate is one of the major tasks faced by cells. | ||
The spontaneous reaction | |||
Depending on the energy source for ATP synthesis, organisms can be classified as: | |||
*[[phototrophy|phototrophic]], which can obtain energy from light. A typical example is provided by the [[light dependent reaction]]s of photosynthesis: in these reactions, excitation of a [[photosystem]] caused by absorption of a light photon markedly lowers its redox potential. Since electron flow tends to occur from low potential species to high potential species, the excited photosystem transfers electrons to higher potential species in an electron transport chain present in the [[thylakoid]] membrane. These electrons eventually reduce NADP<sup>+</sup> to [[NADPH]]. The energy released in the electron transfer steps is used to transport H<sup>+</sup> across the thylakoid membrane, thereby creating a [[proton gradient]] across the thylakoid membrane. The energy stored in this proton gradient can be used to synthesize [[ATP]] from [[ADP]] and [[phosphate]] anion (see [[Chemiosmotic hypothesis]]). | *[[phototrophy|phototrophic]], which can obtain energy from light. A typical example is provided by the [[light dependent reaction]]s of photosynthesis: in these reactions, excitation of a [[photosystem]] caused by absorption of a light photon markedly lowers its redox potential. Since electron flow tends to occur from low potential species to high potential species, the excited photosystem transfers electrons to higher potential species in an electron transport chain present in the [[thylakoid]] membrane. These electrons eventually reduce NADP<sup>+</sup> to [[NADPH]]. The energy released in the electron transfer steps is used to transport H<sup>+</sup> across the thylakoid membrane, thereby creating a [[proton gradient]] across the thylakoid membrane. The energy stored in this proton gradient can be used to synthesize [[ATP]] from [[ADP]] and [[phosphate]] anion (see [[Chemiosmotic hypothesis]]). | ||
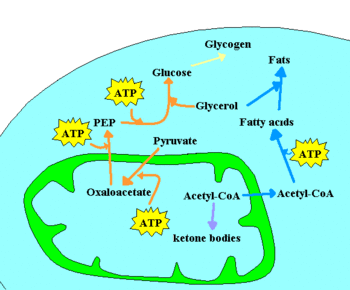
[[Image: Metabolism scheme anabolism.GIF|thumb|350px|A few of the anabolic pathways in a cell. Glucose can be stored as a [[glycogen]] polymer, or synthesized from lower molecular weight precursors. Excess acetyl-CoA can be stored as | [[Image: Metabolism scheme anabolism.GIF|thumb|350px|A few of the anabolic pathways in a cell. Glucose can be stored as a [[glycogen]] polymer, or synthesized from lower molecular weight precursors. Excess acetyl-CoA can be stored as fatty acids, or converted into [[ketone bodies]].]] | ||
*[[chemotroph|chemotrophic]], which obtain energy from chemical reactions. For example, | *[[chemotroph|chemotrophic]], which obtain energy from chemical reactions. For example, [[glucose]] can be oxidized to [[pyruvate]] through [[glycolysis]]. This process yields two molecules of ATP for each molecule of glucose, and releases four electrons, which reduce NAD<sup>+</sup> to [[NADH]]. As the NAD<sup>+</sup> molecules are scarce, the electrons present in NADH must be transferred to another molecule in order to regenerate NAD<sup>+</sup> and to allow the degradation of more glucose molecules. NADH may donate its electrons to pyruvate (or to a pyruvate derivative), in which case a [[fermentation]] is said to occur. Alternatively, the electron acceptor may be a molecule totally unrelated to the metabolic pathway that released the electrons now present in NADH, in which case a [[respiration]] is said to occur. In the presence of NAD<sup>+</sup>, [[pyruvate dehydrogenase]] may decarboxylate pyruvate into [[acetyl-CoA]], a pivotal molecule in metabolism. [[Acetyl-CoA]] can also be formed through [[beta-oxidation|β-oxidation]] of [[fatty acids]] or through the catabolism of amino acids, and is oxidized to CO<sub>2</sub> through the [[Krebs cycle]]. The Krebs cycle releases eight electrons from each acetyl-CoA molecule, which are used to reduce three NAD<sup>+</sup> to three [[NADH]] and one [[FAD]] to FADH<sub>2</sub>. The energy released in electron transfer from NADH and FADH<sub>2</sub> to oxygen (in aerobic organisms) or other electron acceptor (in organisms that perform anaerobic respiration) may be used to create a [[proton gradient]] across a membrane, and to synthesize ATP through dissipation of this gradient (see [[Chemiosmotic hypothesis]]). | ||
In the presence of NAD<sup>+</sup>, [[pyruvate dehydrogenase]] may decarboxylate pyruvate into [[acetyl-CoA]], a pivotal molecule in metabolism. [[Acetyl-CoA]] can also be formed through [[beta-oxidation|β-oxidation]] of [[fatty acids]] or through the catabolism of amino acids, and is oxidized to CO<sub>2</sub> through the [[Krebs cycle]]. The Krebs cycle releases | |||
Organisms can be also be | Organisms can be also be classified, according to the source of reducing power, as: | ||
*organotrophic - use organic compounds (e.g. [[glucose]]) as electron donors. | *organotrophic - use organic compounds (e.g. [[glucose]]) as electron donors. | ||
*[[lithotrophy|lithotrophic]] - use inorganic compounds (e.g. Fe<sup>2+</sup>) as electron donors. | *[[lithotrophy|lithotrophic]] - use inorganic compounds (e.g. Fe<sup>2+</sup>) as electron donors. | ||
Revision as of 06:04, 30 December 2006
Metabolism (from Greek μεταβολισμός "metabolismos") is the biochemical modification of chemical compounds in living organisms and cells. In common use, the word is often used to refer to the basal metabolic rate, the "set point" that each person has in breaking down food energy and building up their own body. Sometimes, in multicellular creatures like humans, it is also used to refer to the overall ingestion of food and excretion of wastes, and the building up of muscles and the growth of the body. This article describes the actual biology of metabolism at a cellular level, which explains just how those processes are carried out. In human and whole animal terms, metabolism also includes the chemical conversion of specific items other than food that may be ingested, like drugs and poisons.
Metabolism involves: (1) anabolism, in which a cell uses energy and chemical reducing power to construct complex molecules, and perform such life functions as creating cellular structure; and (2) catabolism, in which a cell breaks down complex molecules to yield energy and chemical reducing power. Cell metabolism involves complex sequences of controlled chemical reactions called metabolic pathways. Just as the word metabolism can be used to describe processes in a whole organism, such as a person, the terms "anabolism" and "catabolism" can also be used in this way. For example, anabolic processes include building up muscle and adding body weight, and catabolic processes include loss of muscle mass and body fat.
History

The first controlled experiments on human metabolism were published by Santorio Santorio in 1614 in his book Ars de statica medecina, that made him famous throughout Europe. Santorio described his long series of personal experiments: in which he weighed himself in a chair suspended from a steelyard balance (see image), before and after eating, sleeping, working, sex, fasting, depriving from drinking, and excreting. He found that by far the greatest part of the food he took in was lost from the body through perspiratio insensibilis (insensible perspiration). In medicine and the health sciences today, "insensible losses" is still used to refer to the amount of fluid lost from the body through perspiration and processes that (unlike urine or feces) do not yield any obvious portion that can be weighed or measured.
At about the same time Jan Baptist van Helmont made the first observations regarding photosynthesis, when he discovered that plant growth required (almost) no soil nutrients. In the 18th century, Priestley concluded that green plants use CO2 and release O2. In 1804, Nicolas de Saussure discovered that the increase in carbon content of plants (i.e. plant growth) arises from the fixation of atmospheric CO2.Between 1854 and 1864, Louis Pasteur discovered that glucose fermentation is due to microorganisms, and, in 1897, Eduard Buchner proved that cell-free yeast extracts could also perform these reactions, and so the ability to ferment was not limited to entire living creatures (cells)- but included certain portions of their physical contents. Subsequent investigations showed that (with a few exceptions) all living organisms metabolize glucose using the same mechanism.
Overview
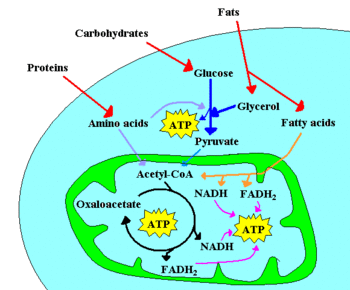
Enzymes present in cells can catalyze a large variety of chemical reactions with exquisite specificity. Often, the chemical reactions needed to synthesize useful cell components involve a positive change in free energy, i.e. they are not spontaneous. In these cases, enzymes may couple the non-spontaneous reaction to a second, very spontaneous, reaction, so that the overall process is spontaneous. The spontaneous reaction usually used to drive metabolism is hydrolysis of ATP in ADP and phosphate anion. Therefore, ATP synthesis from ADP and phosphate is one of the major tasks faced by cells.
Depending on the energy source for ATP synthesis, organisms can be classified as:
- phototrophic, which can obtain energy from light. A typical example is provided by the light dependent reactions of photosynthesis: in these reactions, excitation of a photosystem caused by absorption of a light photon markedly lowers its redox potential. Since electron flow tends to occur from low potential species to high potential species, the excited photosystem transfers electrons to higher potential species in an electron transport chain present in the thylakoid membrane. These electrons eventually reduce NADP+ to NADPH. The energy released in the electron transfer steps is used to transport H+ across the thylakoid membrane, thereby creating a proton gradient across the thylakoid membrane. The energy stored in this proton gradient can be used to synthesize ATP from ADP and phosphate anion (see Chemiosmotic hypothesis).
- chemotrophic, which obtain energy from chemical reactions. For example, glucose can be oxidized to pyruvate through glycolysis. This process yields two molecules of ATP for each molecule of glucose, and releases four electrons, which reduce NAD+ to NADH. As the NAD+ molecules are scarce, the electrons present in NADH must be transferred to another molecule in order to regenerate NAD+ and to allow the degradation of more glucose molecules. NADH may donate its electrons to pyruvate (or to a pyruvate derivative), in which case a fermentation is said to occur. Alternatively, the electron acceptor may be a molecule totally unrelated to the metabolic pathway that released the electrons now present in NADH, in which case a respiration is said to occur. In the presence of NAD+, pyruvate dehydrogenase may decarboxylate pyruvate into acetyl-CoA, a pivotal molecule in metabolism. Acetyl-CoA can also be formed through β-oxidation of fatty acids or through the catabolism of amino acids, and is oxidized to CO2 through the Krebs cycle. The Krebs cycle releases eight electrons from each acetyl-CoA molecule, which are used to reduce three NAD+ to three NADH and one FAD to FADH2. The energy released in electron transfer from NADH and FADH2 to oxygen (in aerobic organisms) or other electron acceptor (in organisms that perform anaerobic respiration) may be used to create a proton gradient across a membrane, and to synthesize ATP through dissipation of this gradient (see Chemiosmotic hypothesis).
Organisms can be also be classified, according to the source of reducing power, as:
- organotrophic - use organic compounds (e.g. glucose) as electron donors.
- lithotrophic - use inorganic compounds (e.g. Fe2+) as electron donors.
Links to subtopics dealing with metabolism
General pathways
Anabolism
Anabolic pathways that create building blocks and compounds from simple precursors:
- Biosynthesis of amino acids and nucleotides
- Glycogenesis
- Gluconeogenesis
- Porphyrin synthesis pathway
- HMG-CoA reductase pathway, leading to cholesterol and isoprenoids.
- Secondary metabolism, metabolic pathways that are not essential for growth, development or reproduction, but that usually have ecological function.
- Photosynthesis
- Light-dependent reaction (light reaction)
- Light-independent reaction (dark reaction)
- Calvin cycle
- Carbon fixation
- Glyoxylate cycle
Catabolism
- Glycolysis
- Glycogenolysis
- Citric acid cycle
- Beta-oxidation of fatty acids
Drug metabolism
Drug metabolism pathways, the modification or degradation of drugs and other xenobiotic compounds through specialized enzyme systems:
Nitrogen metabolism
Nitrogen metabolism includes the pathways for turnover and excretion of nitrogen in organisms as well as the biological processes of the biogeochemical nitrogen cycle:
- Urea cycle, important for excretion of nitrogen as urea.
- Biological nitrogen fixation
- Nitrogen assimilation
- Nitrification
- Denitrification
Other
See also
- Metabolomics
- Metabolome
- Metabolite
- Basal metabolic rate
- Thermic effect of food
- Iron-sulfur world theory, a "metabolism first" theory of the origin of life.
- Biodegradation
- Calorimetry
- Respirometry
- Microbial metabolism
- Metabolic network modelling
- dynamic energy budget
External links
- Interactive Flow Chart of the Major Metabolic Pathways
- Metabolism, Cellular Respiration and Photosynthesis - The Virtual Library of Biochemistry and Cell Biology
- The Biochemistry of Metabolism at Rensselaer Polytechnic Institute
- Flow Chart of Metabolic Pathways at ExPASy
- Santorio Santorio's experiments
- KEGG: Kyoto Encyclopedia of Genes and Genomes