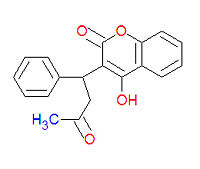
Warfarin
Warfarin (IUPAC name 4-hydroxy-3-(3-oxo-1-phenylbutyl)-2H-chromen-2-one), also widely called coumadin, is a an anticoagulant medication used prophylactically to suppress the formation of embolism and thromboembolism. It was originally designed to be a rat poison. It works as an anticogulant by suppressing the enzyme epoxide reductase in the liver, thereby suppresing the formation of the reduced form of vitamine K epoxide, which is needed for the synthesis of many coagulation factors. As a drug, it is often sold as the sodium salt of warfarin.
Brand names
- Athrombin
- Athrombin-K
- Athrombine-K
- Brumolin
- Co-Rax
- Coumadin
- Coumafen
- Coumafene
- Coumaphen
- Coumaphene
- Coumarins
- Coumefene
- D-Con
- Dethmor
- Dethnel
- Dicusat E
- Frass-Ratron
- Jantoven
- Kumader
- Kumadu
- Kumatox
- Kypfarin
- Latka 42
- Mar-Frin
- Marevan
- Maveran
- Panwarfin
- Place-Pax
- Prothromadin
- RAX
- Rosex
- Sofarin
- Solfarin
- Sorexa Plus
- Temus W
- Tintorane
- Tox-Hid
- Vampirinip II
- Vampirinip III
- Waran
- Warf 42
- Warfarat
- Warfarin Plus
- Warfarin Q
- Warfarine
- Warficide
- Warfilone
- Zoocoumarin
Pharmacokinetics
Absorption
Distribution
Metabolism
Pharmacogenomics
Warfarin activity is determined partially by genetic factors. The American Food and Drug Administration "highlights the opportunity for healthcare providers to use genetic tests to improve their initial estimate of what is a reasonable warfarin dose for individual patients" .[1]
VKORC1
Polymorphisms in the vitamin K epoxide reductase complex 1 (VKORC1) gene explain 30% of the dose variation between patients[2]: particular mutations make VKORC1 less susceptible to suppression by warfarin[3] There are a main haplotypes that explain 25% of variation: low-dose haplotype group (A) and a high-dose haplotype group (B).[4] For the three combinations of the haplotypes, the mean daily maintenance dose of warfarin was:
- A/A: 2.7+/-0.2 mg
- A/B: 4.9+/-0.2 mg
- B/B: 6.2+/-0.3 mg
VKORC1 polymorphisms also explain why African Americans are relatively resistant to warfarin (higher proportion of group B haplotypes), while Asian Americans are more sensitive (higher proportion of group A haplotypes).[4]
CYP2C9
CYP2C9 is an isozyme of cytochrome P450. Polymorphisms of CYP2C9 explain another 10% of variation in warfarin dosing[2], mainly among Caucasian patients as these variants are rare in African American and most Asian populations.[5] A meta-analysis of mainly Caucasian patients found[5]:
- CYP2C9*2 allele:
- present in 12.2% of patients
- mean reduction was in warfarin dose was 0.85 mg (17% reduction)
- relative bleeding risk was 1.91
- CYP2C9*3 allele:
- present in 7.9% of patients
- mean reduction was in warfarin dose was 1.92 mg (37% reduction)
- relative bleeding risk was 1.77
Excretion
Dosage
Loading regimens
| Dosing | Time till anticoagulation (INR=2-3) |
Rate of over-coagulation | |
|---|---|---|---|
| Kovacs 10 mg[6] | Details | 83% by day 5 | 9% within 4 weeks (INR>5) |
| Harrison 10 mg[7] | Details | 63% at 3.5 days | 17% within 3.5 days (INR>4.8) |
| Kovacs 5 mg[6] | Details | 46% by day 5 | 11% within 4 weeks (INR>5) |
| Harrison 5 mg[7] | Details | 80% at 3.5 days | 4% within 4.5 days (INR>4.8) |
Because of warfarin's difficult pharmacokinetics, researchers have proposed algorithms for warfarin loading:
Empiric dosing
- The Harrison 5 mg algorithm reduced excess anticoagulation than the Harrison 10 mg algorithm.[7]
- The Kovacs 10 mg algorithm anticoagulated faster than the Kovacs 5 mg algorithm.[6]
- The Fennerty 10 mg regimen is for urgent anticoagulation[8]
- The Tait 5 mg regimen is for "routine" (low-risk) anticoagulation (summary)[9]
Pharmacogenetic guided dosing
- The Kovacs 10 mg algorithm performed similarly to a pharmacogenetic-guided algorithm.[10]
- Millican derived a pharmacogenetic-based model from a cohort of orthopedic patients. The model included CYP29C and VKORC1 genotype results and predicted 80% of the variation in warfarin doses. It is awaiting validation in larger populations and has not been reproduced in those who require warfarin for other indications.[11]
Adjusting the maintenance dose
Recommendations by the American College of Chest Physicians[12] have been distilled to help manage dose adjustments.[13]
Interactions and contraindications
Some foodstuffs have also been reported to interact with warfarin.[14]
Adverse effects
Patients aged 80 years or more may be especially susceptible to bleeding complications with a rate of 13 bleeds per 100 person-years.[15]
Antagonism and reversal
A detailed table on reversing warfarin are provided in clinical practice guidelines from the American College of Chest Physicians.[12] For patients with an International Normalized Ratio (INR) between 4.5 and 10.0, 1 mg of oral vitamin K is effective.[16]
References
- ↑ FDA Approves Updated Warfarin (Coumadin) Prescribing Information. Retrieved on 2007-08-20.
- ↑ 2.0 2.1 Wadelius M, Chen LY, Downes K, et al (2005). "Common VKORC1 and GGCX polymorphisms associated with warfarin dose". Pharmacogenomics J. 5 (4): 262-70. DOI:10.1038/sj.tpj.6500313. PMID 15883587. Research Blogging.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid14765194 - ↑ 4.0 4.1 Rieder MJ, Reiner AP, Gage BF, et al (2005). "Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose". N. Engl. J. Med. 352 (22): 2285-93. DOI:10.1056/NEJMoa044503. PMID 15930419. Research Blogging.
Cite error: Invalid
<ref>tag; name "pmid15930419" defined multiple times with different content - ↑ 5.0 5.1 Sanderson S, Emery J, Higgins J (2005). "CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis". Genet. Med. 7 (2): 97-104. PMID 15714076. [e]
- ↑ 6.0 6.1 6.2 6.3 Kovacs MJ, Rodger M, Anderson DR, et al (2003). "Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial". Ann. Intern. Med. 138 (9): 714-9. PMID 12729425. [e]
- ↑ 7.0 7.1 7.2 7.3 Harrison L, Johnston M, Massicotte MP, Crowther M, Moffat K, Hirsh J (1997). "Comparison of 5-mg and 10-mg loading doses in initiation of warfarin therapy". Ann. Intern. Med. 126 (2): 133–6. PMID 9005747. [e]
- ↑ Fennerty A, Campbell IA, Routledge PA (1988). "Anticoagulants in venous thromboembolism". BMJ 297 (6659): 1285-8. PMID 3144365. [e]
- ↑ Tait RC, Sefcick A (1998). "A warfarin induction regimen for out-patient anticoagulation in patients with atrial fibrillation". Br. J. Haematol. 101 (3): 450-4. DOI:10.1046/j.1365-2141.1998.00716.x. PMID 9633885. Research Blogging.
- ↑ Anderson JL, Horne BD, Stevens SM, et al (2007). "Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation". Circulation 116 (22): 2563–70. DOI:10.1161/CIRCULATIONAHA.107.737312. PMID 17989110. Research Blogging.
- ↑ Millican E, Jacobsen-Lenzini PA, Milligan PE, et al (2007). "Genetic-based dosing in orthopaedic patients beginning warfarin therapy" 110 (5): 1511-5. DOI:10.1182/blood-2007-01-069609. PMID 17387222. Research Blogging. Online tool based on the study.
- ↑ 12.0 12.1 Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E (2004). "The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy". Chest 126 (3 Suppl): 204S–233S. DOI:10.1378/chest.126.3_suppl.204S. PMID 15383473. Research Blogging.
Cite error: Invalid
<ref>tag; name "pmid15383473" defined multiple times with different content - ↑ Point-of-Care Guides - May 15, 2005 - American Family Physician. Retrieved on 2007-08-20.
- ↑ Holbrook AM, Pereira JA, Labiris R, et al (2005). "Systematic overview of warfarin and its drug and food interactions". Arch. Intern. Med. 165 (10): 1095–106. DOI:10.1001/archinte.165.10.1095. PMID 15911722. Research Blogging.
- ↑ Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S (2007). "Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation". Circulation 115 (21): 2689-96. DOI:10.1161/CIRCULATIONAHA.106.653048. PMID 17515465. Research Blogging. PMID 17515465
- ↑ Crowther MA, Douketis JD, Schnurr T, et al (2002). "Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial". Ann. Intern. Med. 137 (4): 251-4. PMID 12186515. [e]
See also
External links
- Warfarin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).