Steel
Steel is an alloy whose major component is iron, with carbon content between 0.02% and 1.7% by weight, depending on grade. Carbon is the most cost effective alloying material for iron, but many other alloying elements are also used.[1] Carbon and other elements act as a hardening agent, preventing dislocations in the iron atom crystal lattice from sliding past one another. Varying the amount of alloying elements and their distribution in the steel controls qualities such as the hardness, elasticity, ductility, and tensile strength of the resulting steel. Steel with increased carbon content can be made harder and stronger than iron, but is also more brittle. The maximum solubility of carbon in iron is 1.7% by weight, occurring at 1130 degrees Celsius; higher concentrations of carbon or lower temperatures will produce cementite which will reduce the material's strength. Alloys with higher carbon content than this are known as cast iron because of their lower melting point.[1] Steel is also to be distinguished from wrought iron with little or no carbon, usually less than 0.035%. It is common today to talk about 'the iron and steel industry' as if it were a single thing; it is today, but historically they were separate products.
Currently there are several classes of steels in which carbon is replaced with other alloying materials, and carbon, if present, is undesired. A more recent definition is that steels are iron-based alloys that can be plastically formed (pounded, rolled, etc.).
Iron and steel

Iron, like most metals, is not found in the Earth's crust in an elemental state. Iron can be found in the crust only in combination with oxygen or sulfur. Typically Fe2O3—the form of iron oxide (rust) found as the mineral hematite, and FeS2—pyrite (fool's gold). Iron oxide is a soft sandstone-like material with limited uses on its own. Iron is extracted from ore by removing the oxygen by combining it with a preferred chemical partner such as carbon. This process, known as smelting, was first applied to metals with lower melting points. Copper melts at just over 1000 °C, while tin melts around 250 °C. Steel melts at around 1370 °C. Both temperatures could be reached with ancient methods that have been used for at least 6000 years (since the Bronze Age). Since the oxidation rate itself increases rapidly beyond 800 °C, it is important that smelting take place in a low-oxygen environment. Unlike copper and tin, liquid iron dissolves carbon quite readily, so that smelting results in an alloy containing too much carbon to be called steel.
Even in the narrow range of concentrations that make up steel, mixtures of carbon and iron can form into a number of different structures, or allotropes, with very different properties; understanding these is essential to making quality steel. At room temperature, the most stable form of iron is the body-centered cubic (BCC) structure ferrite or α-iron, a fairly soft metallic material that can dissolve only a small concentration of carbon (no more than 0.021 wt% at 910 °C). Above 910 °C ferrite undergoes a phase transition from body-centered cubic to a face-centered cubic (FCC) structure, called austenite or γ-iron, which is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.03 wt% carbon at 1154 °C).[2] As carbon-rich austenite cools, the mixture attempts to revert to the ferrite phase, resulting in an excess of carbon. One way for carbon to leave the austenite is for cementite to precipitate out of the mix, leaving behind iron that is pure enough to take the form of ferrite, and resulting in a cementite-ferrite mixture. Cementite is a stoichiometric phase with the chemical formula of Fe3C. Cementite forms in regions of higher carbon content while other areas revert to ferrite around it. Self-reinforcing patterns often emerge during this process, leading to a patterned layering known as pearlite due to its pearl-like appearance, or the similar but less beautiful bainite.
Perhaps the most important allotrope is martensite, a chemically metastable substance with about four to five times the strength of ferrite. A minimum of 0.4 wt% of carbon is needed in order to form martensite. When the austenite is quenched to form martensite, the carbon is "frozen" in place when the cell structure changes from FCC to BCC. The carbon atoms are much too large to fit in the interstitial vaccancies and thus distort the cell structure into a Body Centered Tetragonal (BCT) structure. Martensite and austenite have an identical chemical composition. As such, it requires extremely little thermal activation energy to form.
The heat treatment process for most steels involves heating the alloy until austenite forms, then quenching the hot metal in water or oil, cooling it so rapidly that the transformation to ferrite or pearlite does not have time to take place. The transformation into martensite, by contrast, occurs almost immediately, due to a lower activation energy.
Martensite has a lower density than austenite, so that the transformation between them results in a change of volume. In this case, expansion occurs. Internal stresses from this expansion generally take the form of compression on the crystals of martensite and tension on the remaining ferrite, with a fair amount of shear on both constituents. If quenching is done improperly, these internal stresses can cause a part to shatter as it cools; at the very least, they cause internal work hardening and other microscopic imperfections. It is common for quench cracks to form when water quenched, although they may not always be visible.
At this point, if the carbon content is high enough to produce a significant concentration of martensite, the result is an extremely hard but very brittle material. Often, steel undergoes further heat treatment at a lower temperature to destroy some of the martensite (by allowing enough time for cementite, etc., to form) and help settle the internal stresses and defects. This softens the steel, producing a more ductile and fracture-resistant metal. Because time is so critical to the end result, this process is known as tempering, which forms tempered steel.
Other materials are often added to the iron-carbon mixture to tailor the resulting properties. Nickel and manganese in steel add to its tensile strength and make austenite more chemically stable, chromium increases the hardness and melting temperature, and vanadium also increases the hardness while reducing the effects of metal fatigue. Large amounts of chromium and nickel (often 18% and 8%, respectively) are added to stainless steel so that a hard oxide forms on the metal surface to inhibit corrosion. Tungsten interferes with the formation of cementite, allowing martensite to form with slower quench rates, resulting in high speed steel. On the other hand sulfur, nitrogen, and phosphorus make steel more brittle, so these commonly found elements must be removed from the ore during processing.
When iron is smelted from its ore by commercial processes, it contains more carbon than is desirable. To become steel, it must be melted and reprocessed to remove the correct amount of carbon, at which point other elements can be added. Once this liquid is cast into ingots, it usually must be "worked" at high temperature to remove any cracks or poorly mixed regions from the solidification process, and to produce shapes such as plate, sheet, wire, etc. It is then heat-treated to produce a desirable crystal structure, and often "cold worked" to produce the final shape. In modern steelmaking these processes are often combined, with ore going in one end of the assembly line and finished steel coming out the other. These can be streamlined by a deft control of the interaction between work hardening and tempering.
Steel Industry
History of steelmaking
Ancient steel
Steel was known in antiquity, and may have been produced by managing the bloomery so that the bloom contained some carbon. Wootz steel produced in India and Sri Lanka from around 300 BC was produced in a wind furnace, blown by the monsoon winds (Juleff 1996). Crucible steel was produced in Merv by 9th to 10th century AD.
Early modern steel
Blister steel
Blister steel, produced by the cementation process was first made in Germany in the early 17th century AD and soon after introduced to England. It was probably produced by Sir Basil Brooke at Coalbrookdale during the 1610s. The raw material for this was bars of wrought iron. During the 17th century it was realised that the best steel came from oregrounds iron from a region of Sweden, north of Stockholm. This was still the usual raw material in the 19th century, almost as long as the process was used.
Crucible steel
Crucible steel is steel that has been melted, with the result that it is more homogeneous than if it had not been. The early modern crucible steel industry resulted from the invention of Benjamin Huntsman in the 1740s. Blister steel (made as above) was melted in a crucible in a furnace, and cast (usually) into ingots.
Styrian steel
Another variety of steel was available in England in the 18th century, known by various names including Cullen (Cologne) steel and German steel. This was made by fining pig iron made from ores in Styria, Austria that were rich in manganese.
Modern steelmaking
The modern era in steelmaking begins with the introduction of Henry Bessemer's Bessemer process in the late 1850s. This enabled steel to be produced in large quantities cheaply, so that mild steel is now used for most purposes for which wrought iron was formerly used. This was only the first of a number of processes. The Gilchrist-Thomas process (or basic Bessemer process) was an improvement to the Bessemer process, lining the converter with a basic material to remove phosphorus. Another was the Siemens-Martin process of open hearth steelmaking.
These were rendered obsolescent by the Linz-Donawitz process of basic oxygen steelmaking, developed in the 1950s, and other oxygen steelmaking processes.
Production today
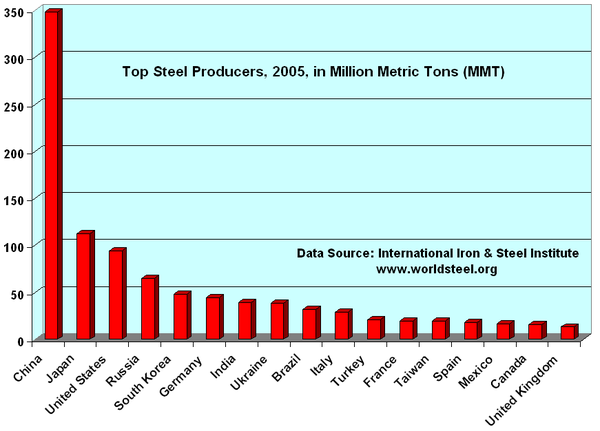
China is currently the largest producer of steel in the world. Steel is currently the most recycled material in the world, the industry estimates that of new metal produced each year some 42.3% is recycled material. All steel that is available is currently recycled, the long service life of steel in applications such as construction means that there is a vast 'store' of steel in use that is recycled as it becomes available. But new metal derived from raw materials is also necessary to make up demand.
Types of steel
Alloy steels were known from antiquity, being nickel-rich iron from meteorites hot-worked into useful products. In a modern sense, alloy steels have been made since the invention of furnaces capable of melting iron, into which other metals could be thrown and mixed.
Here is a table of the types of steel: http://claymore.engineer.gvsu.edu/eod/material/material-4.gif
Historic types
- Damascus steel, which was famous in ancient times for its durability and ability to hold an edge, was created from a number of different materials (some only in traces), essentially a complicated alloy with iron as main component. Some recent studies have suggested that carbon nanotubes were included in its structure, which might explain some of its "legendary" qualities, though given the technology available at that time, they were probably produced more by chance than by design (which might also explain why the process for making damascus steel was "lost" in time and, to this day, has never been successfully duplicated) .
- Blister steel - steel produced by the cementation process
- Crucible steel - steel produced by Benjamin Huntsman's crucible technique
- Styrian Steel, also called 'German steel' or 'Cullen steel' (being traded through Cologne) was made in the Styria in Austria (Roman province of Noricum) by fining cast iron from certain manganese-rich ores.
- Shear steel was blister steel that was broken up, faggotted, heated and welded to produce a more homogeneous product
Contemporary steel
- Carbon steel, composed simply of iron and carbon accounts for 90% of steel production.[1]
- HSLA steel (high strength, low alloy) have small additions (usually <2% by weight) of other elements, typically 1.5% manganese, to provide additional strength for a modest price increase.
- Low alloy steel is alloyed with other elements, usually molybdenum, manganese, chromium, or nickel, in amounts of up to 10% by weight to improve the hardenability of thick sections.[1]
- Stainless steels and surgical stainless steels contain a minimum of 10% chromium, often combined with nickel, to resist corrosion (rust). Some stainless steels are nonmagnetic.
- Tool steels are alloyed with large amounts of tungsten and cobalt or other elements to maximize solution hardening, allow precipitation hardening and improve temperature resistance.[1]
- Cor-ten and related steels weather by acquiring a stable, rusted surface, and so can be used un-painted.
- Advanced high strength steels
- Ferrous superalloys that retain their high mechanical properties at extreme temperatures (i.e. jet engine blades).
- Hadfield steel (after Sir Robert Hadfield) or manganese steel, this contains 12-14% manganese which when abraded forms an incredibly hard skin which resists wearing. Some examples are tank tracks, bulldozer blade edges and cutting blades on the jaws of life.
Though not an alloy, there exists also galvanized steel, which is steel that has gone through the chemical process of being hot-dipped or electroplated in zinc for protection against rust. Finished steel is steel that can be sold without further work or treatment.
Modern steel
- TMT Steel: Thermo mechanically treated Steel: It is one of the latest developments in the history of Steel. The steel manufacturing process is improved and thereby the properties of this steel to suit to the RCC Construction work has been achieved. The steel wires are passed through cold water just after drawing from the extruder. This helps in rapid cooling of the skin and heat starts flowing from the center to the skin once the wire is out of the water. This acts as a heat treatment. The relatively soft core helps in ductility of the steel while the treated skin has good weldability to suit construction requirements.
Production methods
Historical methods
- Bloomery
- Pattern welding
- Catalan forge
- Wootz steel (crucible technique): developed in India, used in the Middle East where it was known as Damascus steel.
- Cementation process used to convert bars of wrought iron into blister steel. This was the main process used in England from the early 17th century.
- Crucible technique, similar to the wootz steel, independently redeveloped in Sheffield by Benjamin Huntsman in c. 1740, and Pavel Anosov in Russia in 1837. Huntsman's raw material was blister steel.
- Puddling
Modern methods
- Electric arc furnace a form of secondary steelmaking from scrap, steel is hard as a result of this, though the process can also use direct-reduced iron
- Production of pig iron using blast furnace
- Converters (steel from pig iron):
- Bessemer process, the first large-scale steel production process for mild steel.
- The Siemens-Martin process, using an open hearth furnace
- Basic oxygen steelmaking
Uses of steel
Iron and steel are required for war, rails, roads, aircrafts, building constructions.
Historically
Steel was expensive and was only used where nothing else would do, particularly for the cutting edge of knives, razors, swords, and other tools where a hard sharp edge was needed. It was also used for springs, including those used in clocks and watches.
Since 1850
Steel has been easier to obtain and much cheaper, and it has replaced wrought iron for a multitude of purposes. It continues to be used in many situations, though the new availability of plastics during the 20th century has meant that it has ceased to be used for some.
Long steel
- Reinforced bars for concrete
- Wires
- Rail tracks
- As girders in building tall modern buildings, bridges
Flat carbon steel
- For the inside and outside body of cars, trains
- Major appliances
Stainless steel
Canadian dimes (since 2000)
- 92% steel
- Weigh 1.75 g
Bibliography
- Duncan Burn; The Economic History of Steelmaking, 1867-1939: A Study in Competition. Cambridge University Press, 1961 online version
- J. C. Carr and W. Taplin; History of the British Steel Industry Harvard University Press, 1962 online version
- Harukiyu Hasegawa; The Steel Industry in Japan: A Comparison with Britain 1996 online version
- H. Lee Scamehorn; Mill & Mine: The Cf&I in the Twentieth Century University of Nebraska Press, 1992 online version
- Warren, Kenneth, Big Steel: The First Century of the United States Steel Corporation, 1901-2001. (University of Pittsburgh Press, 2001) online review
References
G. Juleff, "An ancient wind powered iron smelting technology in Sri Lanka", Nature 379 (3), 60-63 (January, 1996)
- Essays on geology, history, and people
- Early iron in China, Korea, and Japan
- Precursors of the blast furnace
- Early progress in the melting of iron
- Steel City founders
- Various articles on alloys in Historical Metallurgy 19(1) (1985).
- ↑ 1.0 1.1 1.2 1.3 1.4 Ashby, Michael F.; & David R. H. Jones [1986] (1992). Engineering Materials 2 (in English), with corrections. Oxford: Pergamon Press. ISBN 0-08-032532-7.
- ↑ Mittemeijer, E. J.; Slycke, J. T.. Chemical potentials and activities of nitrogen and carbon imposed by gaseous nitriding and carburising atmospheres (PDF). Surface Engineering 1996 Vol. 12 No. 2 156. Retrieved on 2006-08-10.
See also
- Structural steel
- Rolling (metalworking)
- Cold rolling
- Hot rolling
- Steel producers
- Steel mill
- Rolling mill
- Foundry
- Tinplate
- Global steel industry trends
- Pelletizing - Process of creation of iron ore pellets
External links
Template:Commons Template:Wiktionary
- Science Aid: Steel Production and uses of steel, teen study guide
- Extensive picture gallery about all methods of making and shaping of iron and steel in North America and Europe. In German and English.
- International Iron & Steel Institute (IISI)
- Steel Alloys and Their Classification
- steeluniversity.org Free, award-winning e-learning resources on steel technologies for students and steel industry supply chain employees
- Quench hardening of steel
- CIMsteel Integration Standards (CIS/2)
- Steel in 20th-century architecture
- Steel Heat Treating
- Scottish Steel Works History