Stent
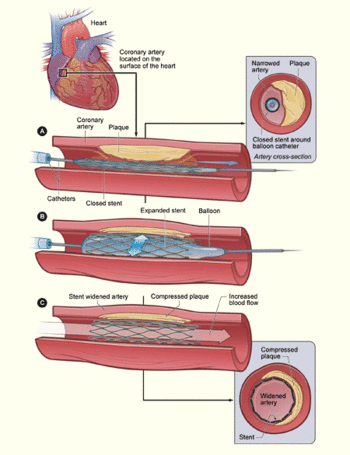
A coronary stent is a small, lattice-shaped, metal tube that is inserted permanently into an artery. The stent helps hold open an artery so that blood can flow through it.[1] It is often used for patients suffering heart attacks or atherosclerosis, a condition in which the arteries become blocked due to plaque build up on the inner walls of arteries, the blood vessels that carry oxygen-rich blood throughout the body. A stent restores blood flow by acting as a permanent scaffold to hold the artery open.
Stents are also used in tubes of the body other than arteries. Ureteral stents are used in a variety of urological treatments, although they are more often temporarily rather than permanently inserted. [2]
Insertion
Stents are typically inserted in the femoral artery in the groin or the brachial artery in the arm and threaded up to the narrowed artery section using a balloon catheter. When the blockage is reached, the balloon is inflated to push the plaque out of the way, expanding the artery. This is referred to as percutaneous transluminal coronary angioplasty (PTCA). Expandable stents are opened as the balloon expands, while other stents are inserted immediately after the PTCA is completed. During the procedure, contrast dye is typically running through the arterial system to guide the insertion of the stent. Following the procedure, the patient must take anti-platelet drugs to avoid thrombosis. Some stents are coated with a time released drug to prevent blood clots. Because stents are inserted into major arteries, a great deal of pressure must be applied to the insertion site to avoid excess bleeding, particularly since the patient has typically been given anti-platelet medications during the procedure.
Risks
Infection, blood clots, or bleeding may occur after stent insertion. Other rare complications of coronary stents include chest pain, heart attack, or tearing of the blood vessel. The stent can move out of place (stent migration). In some cases, plaque can reappear in the stented artery (in-stent restenosis). Drug eluting stents (DES) have additional risks which are being monitored by the FDA since their introduction in 2003-2004.
Drug eluting stents
| Trial(s) | Intervention | Outcome |
|---|---|---|
| STRESS[3] (1994) |
PCI without stent | 20% (6 months)[3] |
| STRESS[3] (1994) SIRIUS[4] (2003) TYPHOON[5] (2006) |
PCI with bare-metal stents | 14%[5]to 14% (6 months)[3] to 21% (9 months)[4] |
| SIRIUS[4] (2003) TYPHOON[5] (2006) SPIRIT IV[6] (2010) |
PCI with first generation DES | 7%[5][6] to 9% (9 months)[4] |
| SPIRIT IV[6] (2010) |
PCI with second generation DES | 4% (one year)[6] |
| SORT OUT III[7] (2010) |
PCI with with zotarolimus DES | 6% (9 months)[7] |
| Resolute All Comers[8] (2010) |
PCI with zotarolimus DES | 8% (9 months)[8] |
Drug-eluting stents further reduce restenosis compared with bare-metal stents; after one year, target-vessel failure was reduced from 14% to 7%.[5] However, drug-eluting stents may increase the rate of delayed restenoses. Delayed restenosis may be prevented by taking aspirin combined with clopidogrel.[9]
The drugs eluted by first generation stents are sirolimus and paclitaxel. Newer drugs are everolimus and zotarolimus. Sirolimus was first approved in the United States of America by the Food and Drug Administration in 2003.[10] Paclitaxel was first approved in the United States of America in 2004.[11] These stents have become one of the best selling devices in medical history, and the worldwide market for drug-eluting coronary stents reached an estimated $4.2 billion in 2004 and is expected to nearly double by 2010.[12]
Two drug eluting stents have dominated the market: the Taxus[13] stent from Boston Scientific and the Cypher[14] stent from Cordis, a Johnson and Johnson company. Taxus elutes the drug paclitaxel, while Cypher elutes sirolimus.
The duration of anti-platelet therapy is uncertain.[15]
References
- ↑ FDA stent webpage.
- ↑ Dyer, RB et al., "Complications of Ureteral Stent Placement", RadioGraphics 22: 1005-1022
- ↑ 3.0 3.1 3.2 3.3 3.4 Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I et al. (1994). "A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators.". N Engl J Med 331 (8): 496-501. PMID 8041414.
- ↑ 4.0 4.1 4.2 4.3 4.4 Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C et al. (2003). "Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery.". N Engl J Med 349 (14): 1315-23. DOI:10.1056/NEJMoa035071. PMID 14523139. Research Blogging.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carrié D et al. (2006). "Sirolimus-eluting versus uncoated stents in acute myocardial infarction.". N Engl J Med 355 (11): 1093-104. DOI:10.1056/NEJMoa062006. PMID 16971716. Research Blogging.
Cite error: Invalid
<ref>tag; name "pmid16971716" defined multiple times with different content - ↑ 6.0 6.1 6.2 6.3 6.4 Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R et al. (2010). "Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease.". N Engl J Med 362 (18): 1663-74. DOI:10.1056/NEJMoa0910496. PMID 20445180. Research Blogging.
- ↑ 7.0 7.1 7.2 Rasmussen K, Maeng M, Kaltoft A, Thayssen P, Kelbaek H, Tilsted HH et al. (2010). "Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial.". Lancet 375 (9720): 1090-9. DOI:10.1016/S0140-6736(10)60208-5. PMID 20231034. Research Blogging.
- ↑ 8.0 8.1 8.2 Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE et al. (2010). "Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents.". N Engl J Med. DOI:10.1056/NEJMoa1004130. PMID 20554978. Research Blogging.
- ↑ Keeley EC, Hillis LD (2007). "Primary PCI for myocardial infarction with ST-segment elevation". N. Engl. J. Med. 356 (1): 47-54. DOI:10.1056/NEJMct063503. PMID 17202455. Research Blogging.
- ↑ Anonymous (2003). Devices@FDA. Food and Drug Administration. Retrieved on 2009-05-02.
- ↑ Anonymous (2004). Devices@FDA. Food and Drug Administration. Retrieved on 2009-05-02.
- ↑ Medical Device Link, Jan./Feb. 2006.
- ↑ Taxus stent webpage.
- ↑ Cypher stent webpage.
- ↑ Park SJ, Park DW, Kim YH, Kang SJ, Lee SW, Lee CW et al. (2010). "Duration of dual antiplatelet therapy after implantation of drug-eluting stents.". N Engl J Med 362 (15): 1374-82. DOI:10.1056/NEJMoa1001266. PMID 20231231. Research Blogging.