User:Milton Beychok/Sandbox
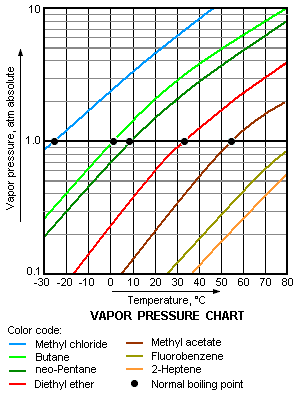
In chemistry and physics, volatility is a term used to characterize the tendency of a substance to vaporize.[1] It is directly related to a substance' s vapor pressure. At a given temperature, a substance with a higher vapor pressure will vaporize more readily than a vapor with a lower vapor pressure.[2][3][4]
Any substance with a significant vapor pressure at temperatures of about 20 – 25 °C (68 – 77 °F) is very often referred to as being volatile.
In common usage, the term applies primarily to liquids. However, it may also be used to characterize the process of sublimation by which certain solid substances such as ammonium chloride (NH4Cl) and dry ice, which is solid carbon dioxide (CO2), change directly from their solid form to a vapor without becoming a liquid.
Any substance with a significant vapor pressure at temperatures of about 20 – 25 °C (68 – 77 °F) is very often referred to as being volatile.
References
- ↑ Note: To vaporize means to become a vapor.
- ↑ Gases and Vapor (University of Kentucky website)
- ↑ James G. Speight (2006). The Chemistry and Technology of Petroleum, 4th Edition. CRC Press. ISBN 0-8493-9067-2.
- ↑ Kister, Henry Z. (1992). Distillation Design, 1st Edition. McGraw-hill. ISBN 0-07-034909-6.