User:Milton Beychok/Sandbox

Atmospheric gases scatter blue light more than other wavelengths, giving the Earth a blue halo when seen from space at an altitude of 181 nautical miles (335 km) above the Earth.
The Earth's atmosphere is an envelope of gas that surrounds the Earth and extends from the Earth's surface out thousands of kilometres, becoming increasingly thinner(less dense with distance but always held in place by Earth's gravitational pull. The atmosphere contains the air we breathe and it holds clouds of moisture (water vapor) that become the water we drink. It protects us from harmful solar radiation and warms the Earth's surface by heat retention. In effect, the atmosphere is an envelope that protects all life on Earth.
The atmosphere is a mixture of gases we call "air". On a dry volume basis, it consists of about 78% nitrogen and 21% oxygen. The remainder of about 1% contains argon, carbon dioxide and very small amounts of other gases. The atmosphere is rarely, if ever, completely dry. Water vapor is almost always present up to about 4% of the total volume. In desert regions, when dry winds are blowing, water vapor in the air will be nearly zero. This climbs in other regions to about 3% on extremely hot and humid days. The upper limit, approaching 4%, is for tropical areas.
The atmosphere has a total mass of about 5 × 1015 metric tons and about 75% of that mass is within 11 kilometres (6.8 miles) from the Earth's surface.
There is no definite boundary between the atmosphere and outer space. It slowly becomes thinner and fades into space. The distance from the Earth's surface that is frequently regarded as the boundary between the atmosphere and outer space is about 100 kilometres (62 miles]) and that boundry is called the Kármán line.
Structure of the atmosphere
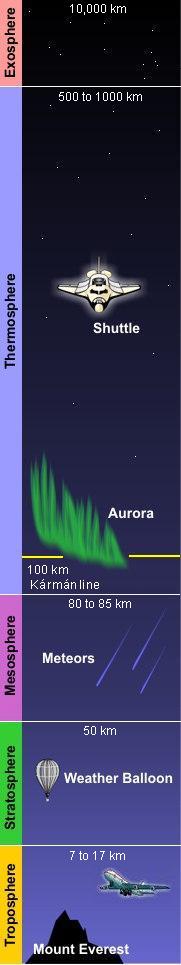
The temperature of the Earth's atmosphere varies with altitude; the mathematical relationship between temperature and altitude varies among five different atmospheric layers (ordered highest to lowest, the ionosphere is part of the thermosphere):
- Exosphere
- From 500 - (Expression error: Unrecognized word "km". 1000) up to 10000 km (Expression error: Missing operand for round. mi ft), contain free-moving particles that may migrate into and out of the magnetosphere or the solar wind.
- Exobase
- Also known as the 'critical level', it is the lower boundary of the exosphere.
- Ionosphere
- The part of the atmosphere that is ionized by solar radiation stretches from 50 to (Expression error: Unrecognized word "km". 1000) and typically overlaps both the exosphere and the thermosphere. It plays an important part in atmospheric electricity and forms the inner edge of the magnetosphere. Because of its charged particles, it has practical importance because it influences, for example, radio propagation on the Earth. It is responsible for auroras.
- Thermopause
- The boundary above the thermosphere, it varies in height from 500 - (Expression error: Unrecognized word "km". 1000).
- Thermosphere
- From 80 - (Expression error: Unrecognized word "km". 85) to over 640 km (Expression error: Missing operand for round. mi ft), temperature increasing with height. The temperature of this layer can rise to 1500 C (Expression error: Missing operand for round. {{{3}}}). The International Space Station orbits in this layer, between 320 and (Expression error: Unrecognized word "km". 380).
- Mesopause
- The temperature minimum at the boundary between the thermosphere and the mesosphere. It is the coldest place on Earth, with a temperature of -100 C (Expression error: Missing operand for round. F K).
- Mesosphere
- From the Greek word "μέσος" meaning middle. The mesosphere extends from about 50 km (Expression error: Missing operand for round. mi ft) to the range of 80 - (Expression error: Unrecognized word "km". 85). Temperature decreases with height, reaching -100 C (Expression error: Missing operand for round. F K) in the upper mesosphere. This is also where most meteors burn up when entering the atmosphere.
- Stratopause
- The boundary between the mesosphere and the stratosphere, typically 50 to (Expression error: Unrecognized word "km". 55). The pressure here is 1/1000th sea level.
- Stratosphere
- From the Latin word "stratus" meaning spreading out. The stratosphere extends from the troposphere's 7 - (Expression error: Unrecognized word "km". 17) range to about 51 km (Expression error: Missing operand for round. mi ft). Temperature increases with height. The stratosphere contains the ozone layer, the part of the Earth's atmosphere which contains relatively high concentrations of ozone. "Relatively high" means a few parts per million—much higher than the concentrations in the lower atmosphere but still small compared to the main components of the atmosphere. It is mainly located in the lower portion of the stratosphere from approximately 15 - (Expression error: Unrecognized word "km". 35) above Earth's surface, though the thickness varies seasonally and geographically.
- Ozone Layer
- Though part of the Stratosphere, the ozone layer is considered as a layer of the Earth's atmosphere in itself because its physical and chemical composition is far different from the Stratosphere. Ozone (O3) in the Earth's stratosphere is created by ultraviolet light striking oxygen molecules containing two oxygen atoms (O2), splitting them into individual oxygen atoms (atomic oxygen); the atomic oxygen then combines with unbroken O2 to create O3. O3 is unstable (although, in the stratosphere, long-lived) and when ultraviolet light hits ozone it splits into a molecule of O2 and an atom of atomic oxygen, a continuing process called the ozone-oxygen cycle. This occurs in the ozone layer, the region from about 10 to (Expression error: Unrecognized word "km". 50) above Earth's surface. About 90% of the ozone in our atmosphere is contained in the stratosphere. Ozone concentrations are greatest between about 20 and (Expression error: Unrecognized word "km". 40), where they range from about 2 to 8 parts per million.
- Tropopause
- The boundary between the stratosphere and troposphere.
- Troposphere
- From the Greek word "τρέπω" meaning to turn or change. The troposphere is the lowest layer of the atmosphere; it begins at the surface and extends to between 7 km (22965.88 ft) at the poles and 17 km (55774.28 ft) at the equator, with some variation due to weather factors. The troposphere has a great deal of vertical mixing because of solar heating at the area. This heating makes air masses less dense so they rise. When an air mass rises, the pressure upon it decreases so it expands, doing work against the opposing pressure of the surrounding air. To do work is to expend energy, so the temperature of the air mass decreases. As the temperature decreases, water vapor in the air mass may condense or solidify, releasing latent heat that further uplifts the air mass. This process determines the maximum rate of decline of temperature with height, called the adiabatic lapse rate. The troposphere contains roughly 80% of the total mass of the atmosphere. Fifty percent of the total mass of the atmosphere is located in the lower 5.6 km (18372.7 ft) of the troposphere.
The average temperature of the atmosphere at the surface of Earth is 14 C (Expression error: Missing operand for round. F K)[1] or 15 C (Expression error: Missing operand for round. F K)[2], depending on the reference.[3] [4][5]
Composition
Filtered air includes trace amounts of many of the chemical elements. Substantial amounts of argon, nitrogen, and oxygen are present as elementary gases. Note the major greenhouse gases: water vapor, carbon dioxide, methane, nitrous oxide, and ozone. Many additional elements from natural sources may be present in tiny amounts in an unfiltered air sample, including contributions from dust, pollen and spores, sea spray, vulcanism, and meteoroids. Various industrial pollutants are also now present in the air, such as chlorine (elementary or in compounds), fluorine (in compounds), elementary mercury, and sulfur (in compounds such as sulfur dioxide [SO2]).
| ppmv: parts per million by volume | |
| Gas | Volume |
|---|---|
| Nitrogen (N2) | 780,840 ppmv (78.084%) |
| Oxygen (O2) | 209,460 ppmv (20.946%) |
| Argon (Ar) | 9,340 ppmv (0.9340%) |
| Carbon dioxide (CO2) | 383 ppmv (0.0383%) |
| Neon (Ne) | 18.18 ppmv (0.001818%) |
| Helium (He) | 5.24 ppmv (0.000524%) |
| Methane (CH4) | 1.745 ppmv (0.0001745%) |
| Krypton (Kr) | 1.14 ppmv (0.000114%) |
| Hydrogen (H2) | 0.55 ppmv (0.000055%) |
| Nitrous oxide (N2O) | 0.3 ppmv (0.00003%) |
| Xenon (Xe) | 0.09 ppmv (9x10−6%) |
| Ozone (O3) | 0.0 to 0.07 ppmv (0%-7x10−6%) |
| Nitrogen dioxide (NO2) | 0.02 ppmv (2x10−6%) |
| Iodine (I) | 0.01 ppmv (1x10−6%) |
| Carbon monoxide (CO) | 0.1 ppmv |
| Ammonia (NH3) | trace |
| Not included in above dry atmosphere: | |
| Water vapor (H2O) | ~0.40% over full atmosphere, typically 1%-4% at surface |
- ↑ Earth's Atmosphere.
- ↑ NASA - Earth Fact Sheet
- ↑ Global Surface Temperature Anomalies.
- ↑ Earth's Radiation Balance and Oceanic Heat Fluxes.
- ↑ Coupled Model Intercomparison Project Control Run.
- ↑ Source for figures: Carbon dioxide, NASA Earth Fact Sheet, (updated 2007.01). Methane, IPCC TAR table 6.1, (updated to 1998). The NASA total was 17 ppmv over 100%, and CO2 was increased here by 15 ppmv. To normalize, N2 should be reduced by about 25 ppmv and O2 by about 7 ppmv.