User:Milton Beychok/Sandbox
Hydrocracking is a catalytic chemical process used in petroleum refineries for converting the high-boiling constituent hydrocarbons in petroleum crude oils to more valuable lower-boiling products such as gasoline, kerosene, jet fuel and diesel oil. The process takes place in a hydrogen-rich atmosphere at elevated temperatures (260 – 425 °C) and pressures (35 – 200 bar).[1][2][3]
Basically, the process cracks the high-boiling, high molecular weight hydrocarbons into lower-boiling, lower molecular weight olefinic and aromatic hydrocarbons and then hydrogenates them. Any sulfur and nitrogen present in the hydrocracking feedstock are, to a large extent, also hydrogenated and form gaseous hydrogen sulfide (H2S) and ammonia (NH3) which are subsequently removed. The result is that the hydrocracking products are essentially free of sulfur and nitrogen impurities and consist mostly of paraffinic hydrocarbons.
Hydrocracking plants are capable of processing a wide variety of feedstocks of different characteristics to produce a broad range of products. They can be designed and operated to maximize the production of a gasoline blending component (called hydrocrackate) or to maximize the production of diesel oil.
As of 2001, there were about 155 hydrocracker units operating worldwide[1] and processing about 4,000,000 barrels (550,000 metric tons) per day of feedstock.[4] As of 2009, The feedstock processing capacity of the hydrocrackers in the United States was 1,740, 000 barrels (238,000 metric tons) per day.[5]
History
Hydrocracking was first developed in Germany as early as 1915 to provide liquid fuels derived from their domestic coal deposits. The first plant that might be considered as a commercial hydrocracking unit began operation in Leuna, Germany in 1927. Similar efforts to convert coal to liquid fuels took place in the Great Britain, France and other countries.[6][7]
Between 1925 and 1930, Standard Oil of New Jersey collaborated with I.G. Farbenindustrie of Germany to develop hydrocracking technology capable of converting heavy petroleum oils into fuels. Such processes required pressures of 200 – 300 bar and temperatures of over 375 °C and were very expensive.
In 1939, Imperial Chemical Industries of Great Britain developed a two-stage hydrocracking process. During World War II (1939 – 1945), two-stage hydrocracking processes played an important role in producing aviation gasoline in Germany, Great Britain and the United States.
After World War II, hydrocracking technology became less important. The availability of petroleum crude oil from the Middle East removed the motivation to convert coal into liquid fuels. Newly developed fluid catalytic cracking processes were much more economical than hydrocracking for converting high-boiling petroleum oils to fuels.
In the early 1960s, hydrocracking become economical for a number of reasons:
- The automobile industry began manufacturing higher-performing automobiles that required high-octane gasoline.
- Fluid catalytic cracking expanded rapidly to meet the demand for high-octane gasoline. However, fluid catalytic cracking, in additon to producing gasoline, produces a by-product high-boiling oil called cycle oil that is very difficult to recycle for further cracking. However, hydrocracking can crack that cycle oil.
- The switch from railroad steam engines to diesel engines and the introduction of commercial jet aircraft in the 1950's increased the demand for diesel oil and for jet fuel. The flexibility of hydrocracking to produce either gasoline, jet fuel or diesel oil made it desirable for petroleum refineries to install hydrocrackers.
- Zeolite-based catalysts, developed and commercialized during the period from about 1964 to 1966, performed much better than the earlier catalysts. Most importantly, they permitted operation at lower pressures than possible with the earlier catalysts. The higher performance and lower operating pressures made possible by the new catalysts resulted in significantly more economical hydrocrackers.
Hydrocracking enjoyed rapid growth in the United States during the late 1960s and the early 1970s. By the mid-1970s, hydrocracking had become a mature process and its growth began to moderate. From then on, hydrocracking growth in the United States proceeded at a slow pace. However, at the same time, hydrocracking enjoyed significant growth in Europe, the Asia-Pacific region and the Middle East.
Process configurations and a typical flow diagram
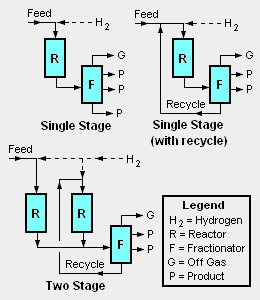
There are a good many different proprietary hydrocracker designs available for use under license as is the case for many of the other processes used in petroleum refineries. There are also a number of different hydrocracker process equipment configurations, the most common of which are depicted in the adjacent diagram:
- Single stage, once through hydrocracker: This configuration uses only one reactor and any uncracked residual hydrocarbon oil from the bottom of the reaction product fractionation (distillation) tower is not recycled for further cracking. For single stage hydrocracking, either the feedstock must first be hydrotreated to remove ammonia and hydrogen sulfide or the catalyst used in the single reactor must be capable of both hydrotreating and hydrocracking.[1]
- Single stage hydrocracker with recycle: This is the most commonly used configuration. The uncracked residual hydrocarbon oil from the bottom of reaction product fractionation tower is recycled back into the single reactor for further cracking. Again, for single stage hydrocracking, either the feedstock must first be hydrotreated to remove ammonia and hydrogen sulfide or the catalyst used in the single reactor must be capable of both hydrotreating and hydrocracking.[1]
- Two stage hydrocracker: This configuration uses two reactors and the residual hydrocarbon oil from the bottom of reaction product fractionation tower is recycled back into the second reactor for further cracking. Since the the first stage reactor accomplishes both hydrotreating and hydrocracking, the second stage reactor feed is virtually free of ammonia and hydrogen sulfide. This permits the use of high performance noble metal (palladium, platinum) catalysts which are susceptible to poisoning by sulfur or nitrogen compounds.[1]
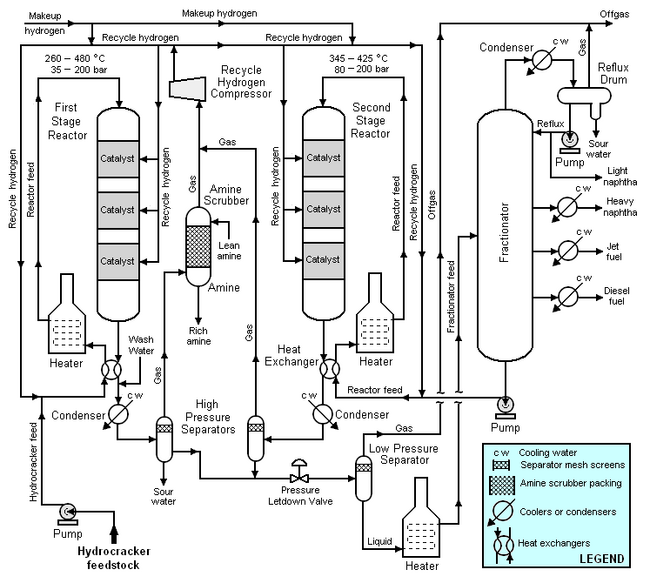
Typical flow diagram of a two stage hydrocracker
The high-boiling, high molecular weight hydrocarbons used as feedstocks for catalytic hydrocrackers include what are commonly referred to as atmospheric gas oil from atmospheric crude oil distillation units, vacuum gas oil from vacuum distillation units, and cycle oil from fluid catalytic cracking units. For describing the hydrocracking process depicted in the typical flow diagram below, the feedstock will be referred to as simply gas oil.
The gas oil from the feedstock pump is mixed with a stream of high-pressure hydrogen and then flows through a heat exchanger where it is heated by the hot effluent reaction products from the hydrocracker's first stage reactor. The feedstock is then heated further in a fuel-fired heater before it enters the top of first stage reactor and flows downward through three beds of catalyst. The temperature and pressure conditions in the first stage reactor depend upon the specific licensed hydrocracker design, the feedstock properties, the desired products, the catalyst being used and other variables. As a broad generality, the pressure in the first stage reactor may range from 35 to 200 bar and the temperature may range from 260 to 480 °C.
After the effluent reaction product stream from the reactor bottom is cooled by the incoming gas oil feedstock, it is injected with wash water, partially condensed in a water-cooled condenser and routed into a high-pressure vapor-liquid separator for separation into three phases: gas, hydrocarbon liquid and water. Sulfur and nitrogen compounds in the gas oil feedstock are converted into gaseous hydrogen sulfide and ammonia by the hydrogenation that takes place in the first stage reactor. The purpose of the wash water is to dissolve the hydrogen and ammonia gases present in the first stage reaction product stream and remove them from the process as an aqueous solution of ammonium hydrosulfide (NH4HS) referred to as sour water. The hydrocarbon liquid phase from the high-pressure separator flows through a pressure letdown (i.e., pressure reduction) valve and into a low-pressure separator. The sour water from the high-pressure separator is typically routed to a sour water stripper elsewhere in the petroleum refinery.
The gas from the high-pressure separator is routed through an amine scrubber to absorb any remaining hydrogen sulfide. The rich amine (containing the absorbed hydrogen sulfide) is typically routed to a central amine gas treating unit elsewhere in the refinery.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 David S.J. Jones and Peter P.Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9.
- ↑ James H. Gary and Glenn E. Handwerk (1984). Petroleum Refining: Technology and Economics, 2nd Edition. Marcel Dekker. ISBN 0-8247-7150-8.
- ↑ Editorial Staff (November 2002). "Refining Processes 2002". Hydrocarbon Processing : pages 115 – 117.
- ↑ J.G. Speight and Baki Ozum (2002). Petroleum Refining Processes. Marcel Dekker. ISBN 0-8247-0599-8.
- ↑ Number and Capacity of Petroleum Refineries From the website of the U.S. Energy Information Administration (U.S. EIA), using the drop down Data Series menu to select Catalytic Hydrocracking Charge Capacity.
- ↑ Julius Scherzer and A.J. Gruia (1996). Hydrocracking Science and Technology, 1st Edition. CRC Press. ISBN 0-8247-9760-4. (This book was the source for most of the History section of this article)
- ↑ Hydrocracking (From the website of Chemical Engineering Resources, which also provided some of this historical information)
- Naveen Bhutani, Ajay K. Ray and G.P. Rangaiah (2006). "Modeling, Simulation and Multi-objective Optimization of an Industrial Hydrocracking Unit". Ind. Eng. Res. 45 (4): pages 1354 – 1372.